Using a chemical equation to find moles of product from moles of reactant Wine goes bad soon after opening because the ethanol (CH3CH2OH) dissolved in it reacts with oxygen (02) gas to form water and aqueous acetic acid (CH3COOH), the main ingredient in vinegar. Calculate the moles of oxygen needed to produce 0.065 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. G 0/5
Using a chemical equation to find moles of product from moles of reactant Wine goes bad soon after opening because the ethanol (CH3CH2OH) dissolved in it reacts with oxygen (02) gas to form water and aqueous acetic acid (CH3COOH), the main ingredient in vinegar. Calculate the moles of oxygen needed to produce 0.065 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. G 0/5
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 56A
Related questions
Question
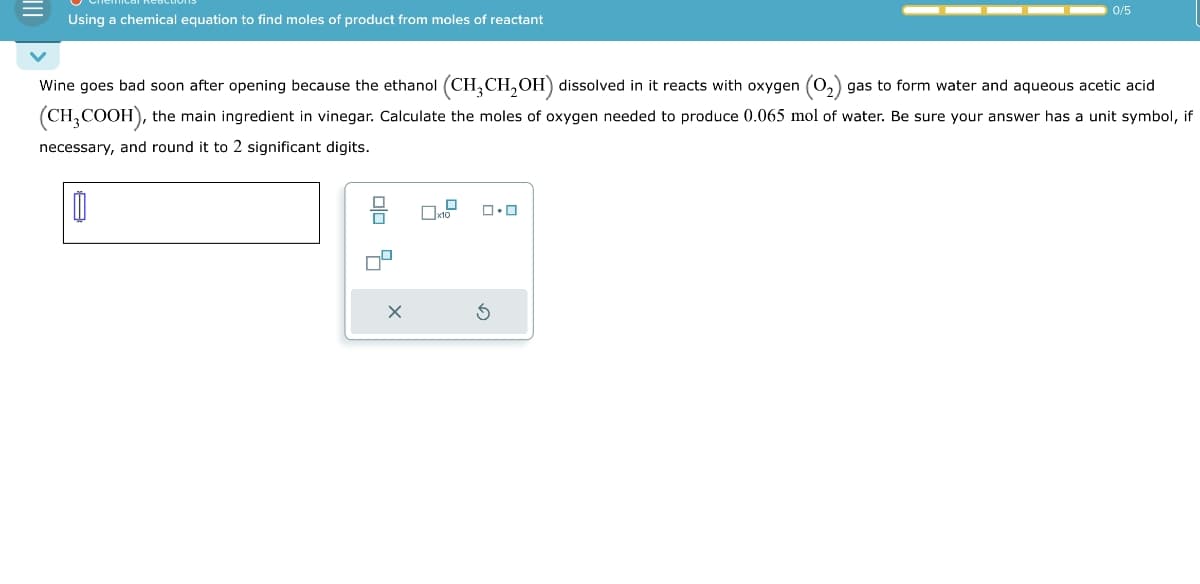
Transcribed Image Text:Using a chemical equation to find moles of product from moles of reactant
Wine goes bad soon after opening because the ethanol (CH3CH2OH) dissolved in it reacts with oxygen (02) gas to form water and aqueous acetic acid
(CH3COOH), the main ingredient in vinegar. Calculate the moles of oxygen needed to produce 0.065 mol of water. Be sure your answer has a unit symbol, if
necessary, and round it to 2 significant digits.
G
0/5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning