Using a pipette, add 2 mL of suspended DEAE SEPHAROSE (anion) resin to a chromatographic column. Repeat the same step to another column. Make sure the column valve is closed. Let it settle by gravity until you see a defined and settled top. 2. Open the column valve and let the preservative liquid slowly drip through until there remains only tiny bit on top of the column. Close the column valve. Repeat the same step to another column. 3. Add 10 mL of equilibration buffer and let it pass through the column (to wash out preservative). Discard as waste. Repeat the same step to another column. Close the valve when 8 mL of the equilibration buffer pass through the column. 4. Label 2 sets of test tubes 1-6. Label one set for lysozyme and the other set for albumin. 5. Using a micropipette, gently add 200 µL of lysozyme solution to the top of the first column (without disturbing the top of the bed). Open the column valve and allow the sample to "load" into the column. Collect the flow through into tube #1 for the first protein set. Close the column valve before the column runs dry. Repeat the same procedure on the second column by adding 200µL of albumin solution. 6. Add another 2 mL of equilibration buffer and let it pass through the column slowly. Collect the flow through into test tube # 2. 7. Repeat step 6, three more times for the column, collecting fractions slowly into test tubes #3, 4, 5 respectively. 8. Add 2 mL of elution (high salt) buffer to the top of the column. Open the valve. 9. As the elution slowly comes through, collect the final 2 mL sample in test tube #6. Close the column valve just before the column runs dry. 10. Test each fraction in your "run" with Bradford reagent to determine the presence of protein, by following the directions below. Blue color indicates the presence of protein. a) Turn on the spectrophotometer. Let it warm up 15 minutes. b) Add 3.0 mL of Bradford reagent to each of the test tubes 1-6 for each of the two protein sets. c) Cover each of the tubes with a tiny square of Parafilm. d) Invert each tube 3x to mix. Be gentle. Don't allow the solution to foam up. Don't mix up the Parafilm covers. Wait for 2 minutes before taking absorbance readings. e) Transfer the solutions to proper spec cuvettes (plastic) f) Make up a blank with 2.0 mL of equilibration buffer and 3.0 mL of Bradford reagent. Mix. g) Set the Spec to 595 nm. Collect the baseline using the blank following the instructions for the spectrophotometer. h) Wipe the outside of each spec tube and read the absorbance of each set of samples 1-6
Using a pipette, add 2 mL of suspended DEAE SEPHAROSE (anion) resin to a chromatographic column. Repeat the same step to another column. Make sure the column valve is closed. Let it settle by gravity until you see a defined and settled top. 2. Open the column valve and let the preservative liquid slowly drip through until there remains only tiny bit on top of the column. Close the column valve. Repeat the same step to another column. 3. Add 10 mL of equilibration buffer and let it pass through the column (to wash out preservative). Discard as waste. Repeat the same step to another column. Close the valve when 8 mL of the equilibration buffer pass through the column. 4. Label 2 sets of test tubes 1-6. Label one set for lysozyme and the other set for albumin. 5. Using a micropipette, gently add 200 µL of lysozyme solution to the top of the first column (without disturbing the top of the bed). Open the column valve and allow the sample to "load" into the column. Collect the flow through into tube #1 for the first protein set. Close the column valve before the column runs dry. Repeat the same procedure on the second column by adding 200µL of albumin solution. 6. Add another 2 mL of equilibration buffer and let it pass through the column slowly. Collect the flow through into test tube # 2. 7. Repeat step 6, three more times for the column, collecting fractions slowly into test tubes #3, 4, 5 respectively. 8. Add 2 mL of elution (high salt) buffer to the top of the column. Open the valve. 9. As the elution slowly comes through, collect the final 2 mL sample in test tube #6. Close the column valve just before the column runs dry. 10. Test each fraction in your "run" with Bradford reagent to determine the presence of protein, by following the directions below. Blue color indicates the presence of protein. a) Turn on the spectrophotometer. Let it warm up 15 minutes. b) Add 3.0 mL of Bradford reagent to each of the test tubes 1-6 for each of the two protein sets. c) Cover each of the tubes with a tiny square of Parafilm. d) Invert each tube 3x to mix. Be gentle. Don't allow the solution to foam up. Don't mix up the Parafilm covers. Wait for 2 minutes before taking absorbance readings. e) Transfer the solutions to proper spec cuvettes (plastic) f) Make up a blank with 2.0 mL of equilibration buffer and 3.0 mL of Bradford reagent. Mix. g) Set the Spec to 595 nm. Collect the baseline using the blank following the instructions for the spectrophotometer. h) Wipe the outside of each spec tube and read the absorbance of each set of samples 1-6
Chapter16: Testing Testing
Section: Chapter Questions
Problem 3MC
Related questions
Question
What is the expected outcome of the ion exchange chromatography using lysozyme as outlined above? Will the protein be separated successfully or not using the materials stated in the above? Provide a brief explanation to your reasoning
please answer correctly, not written assigment
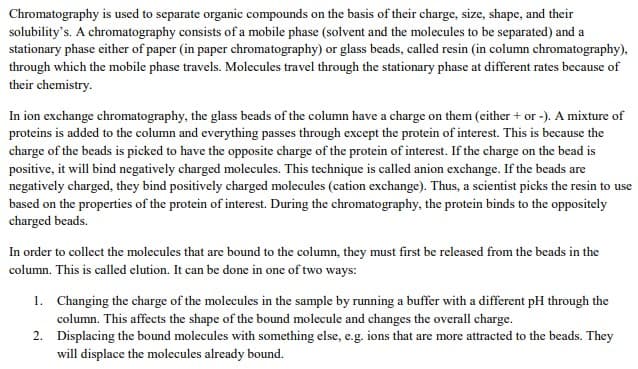
Transcribed Image Text:Chromatography is used to separate organic compounds on the basis of their charge, size, shape, and their
solubility's. A chromatography consists of a mobile phase (solvent and the molecules to be separated) and a
stationary phase either of paper (in paper chromatography) or glass beads, called resin (in column chromatography),
through which the mobile phase travels. Molecules travel through the stationary phase at different rates because of
their chemistry.
In ion exchange chromatography, the glass beads of the column have a charge on them (either + or -). A mixture of
proteins is added to the column and everything passes through except the protein of interest. This is because the
charge of the beads is picked to have the opposite charge of the protein of interest. If the charge on the bead is
positive, it will bind negatively charged molecules. This technique is called anion exchange. If the beads are
negatively charged, they bind positively charged molecules (cation exchange). Thus, a scientist picks the resin to use
based on the properties of the protein of interest. During the chromatography, the protein binds to the oppositely
charged beads.
In order to collect the molecules that are bound to the column, they must first be released from the beads in the
column. This is called elution. It can be done in one of two ways:
1. Changing the charge of the molecules in the sample by running a buffer with a different pH through the
column. This affects the shape of the bound molecule and changes the overall charge.
2. Displacing the bound molecules with something else, e.g. ions that are more attracted to the beads. They
will displace the molecules already bound.

Transcribed Image Text:Using a pipette, add 2 mL of suspended DEAE SEPHAROSE (anion) resin to a chromatographic column.
Repeat the same step to another column. Make sure the column valve is closed. Let it settle by gravity
until you see a defined and settled top.
2. Open the column valve and let the preservative liquid slowly drip through until there remains only tiny
bit on top of the column. Close the column valve. Repeat the same step to another column.
3. Add 10 mL of equilibration buffer and let it pass through the column (to wash out preservative).
Discard
as waste. Repeat the same step to another column. Close the valve when 8 mL of the equilibration buffer
pass through the column.
4. Label 2 sets of test tubes 1-6. Label one set for lysozyme and the other set for albumin.
5. Using a micropipette, gently add 200 µL of lysozyme solution to the top of the first column (without
disturbing the top of the bed). Open the column valve and allow the sample to "load" into the column.
Collect the flow through into tube #1 for the first protein set. Close the column valve before the column
runs dry. Repeat the same procedure on the second column by adding 200µL of albumin solution.
6. Add another 2 mL of equilibration buffer and let it pass through the column slowly. Collect the flow
through into test tube # 2.
7. Repeat step 6, three more times for the column, collecting fractions slowly into test tubes #3, 4, 5
respectively.
8. Add 2 mL of elution (high salt) buffer to the top of the column. Open the valve.
9. As the elution slowly comes through, collect the final 2 mL sample in test tube #6. Close the column
valve just before the column runs dry.
10. Test each fraction in your "run" with Bradford reagent to determine the presence of protein, by
following the directions below. Blue color indicates the presence of protein.
a) Turn on the spectrophotometer. Let it warm up 15 minutes.
b) Add 3.0 mL of Bradford reagent to each of the test tubes 1-6 for each of the two protein sets.
c) Cover each of the tubes with a tiny square of Parafilm.
d) Invert each tube 3x to mix. Be gentle. Don't allow the solution to foam up. Don't mix up the Parafilm
covers. Wait for 2 minutes before taking absorbance readings.
e) Transfer the solutions to proper spec cuvettes (plastic)
f) Make up a blank with 2.0 mL of equilibration buffer and 3.0 mL of Bradford reagent. Mix.
g) Set the Spec to 595 nm. Collect the baseline using the blank following the instructions for the
spectrophotometer.
h) Wipe the outside of each spec tube and read the absorbance of each set of samples 1-6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Comprehensive Medical Assisting: Administrative a…
Nursing
ISBN:
9781305964792
Author:
Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy Correa
Publisher:
Cengage Learning



Comprehensive Medical Assisting: Administrative a…
Nursing
ISBN:
9781305964792
Author:
Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy Correa
Publisher:
Cengage Learning
