Which of the following statements are FALSE? Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE V When S is converted to P by an enzyme the reaction equilibrium is shifted to the right. Enzymes are distinguished from inorganic catalysts because enzymes display specificity toward their reactants i.e substrates and plain catalysts do not. Vmax for an enzyme-catalyzed reaction is twice the rate observed when the concentration of substrate is equal to the Km: The Lineweaver-Burk plot is used to solve graphically the ratio of products to reactants for any starting substrate d concentration. An enzyme-catalyzed reaction was carried out with the substrate concentration initially 1000 times greater than the Km for that substrate. After 9 minutes 1 % of the substrate had been converted to product and the amount of product formed e in the reaction mixture was 12 micromoles. In a separate experiment one-third as much enzyme and twice as much substrate was used. The amount of time required to make 12 micromoles of product is 27 minutes. f In competitive inhibition an inhibitor binds only to the ES complex. For many enzymes the slowest (rate-limiting) step is the reaction that actually releases the product. Under these conditions k2 can be ignored and Km becomes equivalent to the dissociation constant for the ES complex. The difference in free energy content (AG) between substrate (or reactant) and product for a reaction reflects the relative amounts of each compound present at equilibrium. i The rate of conversion from substrate to product depends on the free-energy (G) difference between them.
Which of the following statements are FALSE? Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE V When S is converted to P by an enzyme the reaction equilibrium is shifted to the right. Enzymes are distinguished from inorganic catalysts because enzymes display specificity toward their reactants i.e substrates and plain catalysts do not. Vmax for an enzyme-catalyzed reaction is twice the rate observed when the concentration of substrate is equal to the Km: The Lineweaver-Burk plot is used to solve graphically the ratio of products to reactants for any starting substrate d concentration. An enzyme-catalyzed reaction was carried out with the substrate concentration initially 1000 times greater than the Km for that substrate. After 9 minutes 1 % of the substrate had been converted to product and the amount of product formed e in the reaction mixture was 12 micromoles. In a separate experiment one-third as much enzyme and twice as much substrate was used. The amount of time required to make 12 micromoles of product is 27 minutes. f In competitive inhibition an inhibitor binds only to the ES complex. For many enzymes the slowest (rate-limiting) step is the reaction that actually releases the product. Under these conditions k2 can be ignored and Km becomes equivalent to the dissociation constant for the ES complex. The difference in free energy content (AG) between substrate (or reactant) and product for a reaction reflects the relative amounts of each compound present at equilibrium. i The rate of conversion from substrate to product depends on the free-energy (G) difference between them.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Solve all parts otherwise I will downvote
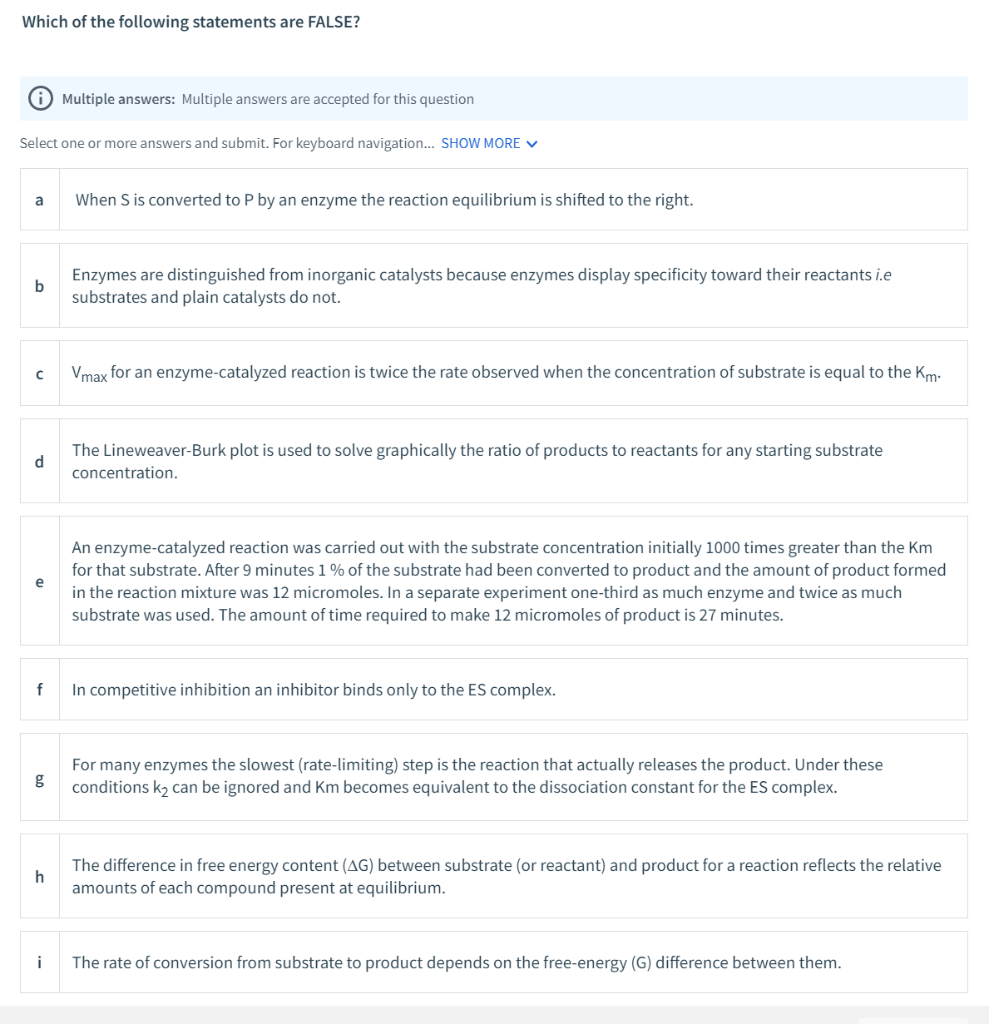
Transcribed Image Text:Which of the following statements are FALSE?
Multiple answers: Multiple answers are accepted for this question
Select one or more answers and submit. For keyboard navigation... SHOW MORE V
When S is converted to P by an enzyme the reaction equilibrium is shifted to the right.
Enzymes are distinguished from inorganic catalysts because enzymes display specificity toward their reactants i.e
substrates and plain catalysts do not.
Vmax for an enzyme-catalyzed reaction is twice the rate observed when the concentration of substrate is equal to the Km:
The Lineweaver-Burk plot is used to solve graphically the ratio of products to reactants for any starting substrate
d
concentration.
An enzyme-catalyzed reaction was carried out with the substrate concentration initially 1000 times greater than the Km
for that substrate. After 9 minutes 1 % of the substrate had been converted to product and the amount of product formed
e
in the reaction mixture was 12 micromoles. In a separate experiment one-third as much enzyme and twice as much
substrate was used. The amount of time required to make 12 micromoles of product is 27 minutes.
f
In competitive inhibition an inhibitor binds only to the ES complex.
For many enzymes the slowest (rate-limiting) step is the reaction that actually releases the product. Under these
conditions k2 can be ignored and Km becomes equivalent to the dissociation constant for the ES complex.
The difference in free energy content (AG) between substrate (or reactant) and product for a reaction reflects the relative
amounts of each compound present at equilibrium.
i
The rate of conversion from substrate to product depends on the free-energy (G) difference between them.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON