A hydrocarbon system is flashed in a drum at 325 psig and 300 K. Assume that the system obeys Raoult's Law and Antoine's equation is sufficient to model their vapor pressures. Antoine's Coefficients B 405.4200 663.7000 803.8100 935.8600 1075.7800 Component Methane Ethane Propane Butane Pentane A 6.6956 6.8345 6.8040 6.8090 6.8763 Antoine's equation is given by: C 267.7770 256.4700 246.9900 238.7300 233.2050 B log psat (mmHg) = AT (°C) + C Feed Composition 0.1300 0.1700 0.3500 0.2900 0.0600 a. Calculate the bubble P and dew P of the said feed mixture. b. Determine the composition of the vapor and liquid streams and the fraction of feed vaporized. c. If the feed rate is 1000 mol/h, what are the flowrates of the liquid and vapor streams?
A hydrocarbon system is flashed in a drum at 325 psig and 300 K. Assume that the system obeys Raoult's Law and Antoine's equation is sufficient to model their vapor pressures. Antoine's Coefficients B 405.4200 663.7000 803.8100 935.8600 1075.7800 Component Methane Ethane Propane Butane Pentane A 6.6956 6.8345 6.8040 6.8090 6.8763 Antoine's equation is given by: C 267.7770 256.4700 246.9900 238.7300 233.2050 B log psat (mmHg) = AT (°C) + C Feed Composition 0.1300 0.1700 0.3500 0.2900 0.0600 a. Calculate the bubble P and dew P of the said feed mixture. b. Determine the composition of the vapor and liquid streams and the fraction of feed vaporized. c. If the feed rate is 1000 mol/h, what are the flowrates of the liquid and vapor streams?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
pls help me out
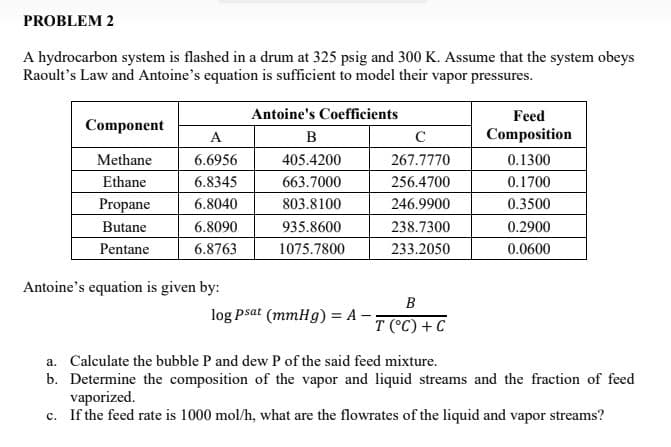
Transcribed Image Text:PROBLEM 2
A hydrocarbon system is flashed in a drum at 325 psig and 300 K. Assume that the system obeys
Raoult's Law and Antoine's equation is sufficient to model their vapor pressures.
Antoine's Coefficients
B
405.4200
663.7000
803.8100
935.8600
1075.7800
Component
Methane
Ethane
Propane
Butane
Pentane
A
6.6956
6.8345
6.8040
6.8090
6.8763
Antoine's equation is given by:
log psat (mmHg) = A -
C
267.7770
256.4700
246.9900
238.7300
233.2050
B
T (°C) + C
Feed
Composition
0.1300
0.1700
0.3500
0.2900
0.0600
a. Calculate the bubble P and dew P of the said feed mixture.
b. Determine the composition of the vapor and liquid streams and the fraction of feed
vaporized.
c. If the feed rate is 1000 mol/h, what are the flowrates of the liquid and vapor streams?
Expert Solution
Step by step
Solved in 9 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The