Using the appropriate bond energies, calculate the heat of reaction AH for the following reaction: 2 H-Br - H−H + Br-Br You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. Note: For clarity, all lone pairs have been omitted from the molecular structures. mol 5
Using the appropriate bond energies, calculate the heat of reaction AH for the following reaction: 2 H-Br - H−H + Br-Br You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. Note: For clarity, all lone pairs have been omitted from the molecular structures. mol 5
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.12: Over The Hill: Reversing Reactions
Problem 4E
Related questions
Question
Payalben
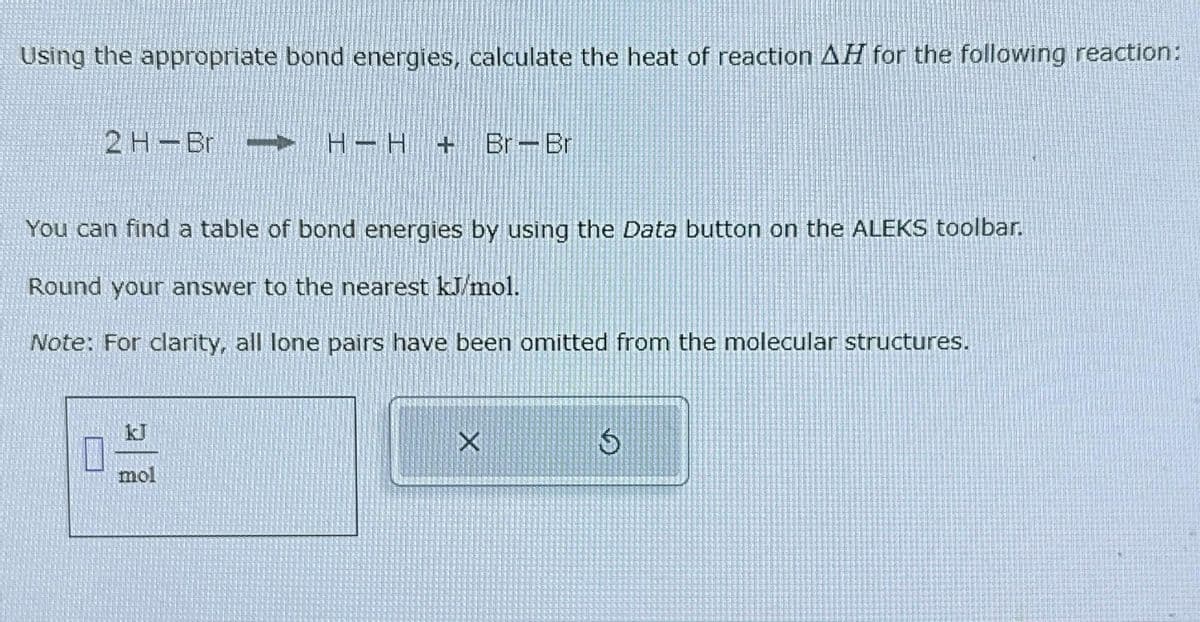
Transcribed Image Text:Using the appropriate bond energies, calculate the heat of reaction AH for the following reaction:
2 H-Br - H−H + Br-Br
You can find a table of bond energies by using the Data button on the ALEKS toolbar.
Round your answer to the nearest kJ/mol.
Note: For clarity, all lone pairs have been omitted from the molecular structures.
mol
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning