Using the evidence above, list the chemical and physical properties of Phosphorous. Physical Properties Chemical Properties 1. 1. 2. 3. 3. 4. 4. 2.
Using the evidence above, list the chemical and physical properties of Phosphorous. Physical Properties Chemical Properties 1. 1. 2. 3. 3. 4. 4. 2.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter7: Ionic Compounds And Metals
Section: Chapter Questions
Problem 122A
Related questions
Question

Transcribed Image Text:Based on what you have read and without looking at the periodic table, is phosphorous more likely to be
considered a metal or a nonmetal? Explain using CLAIM, EVIDENCE, REASONING.
CLAIM
EVIDENCE
REASONING
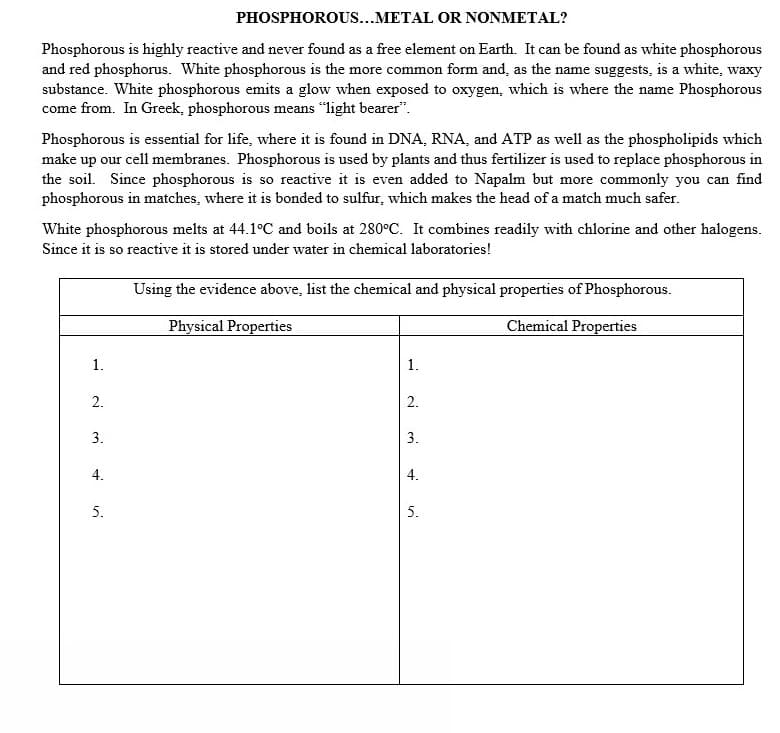
Transcribed Image Text:PHOSPHOROUS...METAL OR NONMETAL?
Phosphorous is highly reactive and never found as a free element on Earth. It can be found as white phosphorous
and red phosphorus. White phosphorous is the more common form and, as the name suggests, is a white, waxy
substance. White phosphorous emits a glow when exposed to oxygen, which is where the name Phosphorous
come from. In Greek, phosphorous means "light bearer".
Phosphorous is essential for life, where it is found in DNA, RNA, and ATP as well as the phospholipids which
make up our cell membranes. Phosphorous is used by plants and thus fertilizer is used to replace phosphorous in
the soil. Since phosphorous is so reactive it is even added to Napalm but more commonly you can find
phosphorous in matches, where it is bonded to sulfur, which makes the head of a match much safer.
White phosphorous melts at 44.1°C and boils at 280°C. It combines readily with chlorine and other halogens.
Since it is so reactive it is stored under water in chemical laboratories!
Using the evidence above, list the chemical and physical properties of Phosphorous.
Physical Properties
Chemical Properties
1.
2.
3.
4.
4.
5.
1.
2.
5.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning