Using the given standard enthalpies of formation in the table to calculate A Horxn for this reaction. 2 CH3OH(1) + O2(g) → CH3COOH(I) + 2 H2O(1) AHof CH3OH(1) -238 kJ/mol CH3COOH(1) -487 kJ/mol H2O(1) -286 kJ/mol H₂O(g) -241 kJ/mol
Using the given standard enthalpies of formation in the table to calculate A Horxn for this reaction. 2 CH3OH(1) + O2(g) → CH3COOH(I) + 2 H2O(1) AHof CH3OH(1) -238 kJ/mol CH3COOH(1) -487 kJ/mol H2O(1) -286 kJ/mol H₂O(g) -241 kJ/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.10: Standard Formation Enthalpies
Problem 4.14PSP: Use data from Table 4.2 to calculate the standard combustion enthalpy for conversion of sulfur...
Related questions
Question
i know the answer is -583kJ but I did not understand why. I would appreciate any help on how to solve this step by step, thank you :)
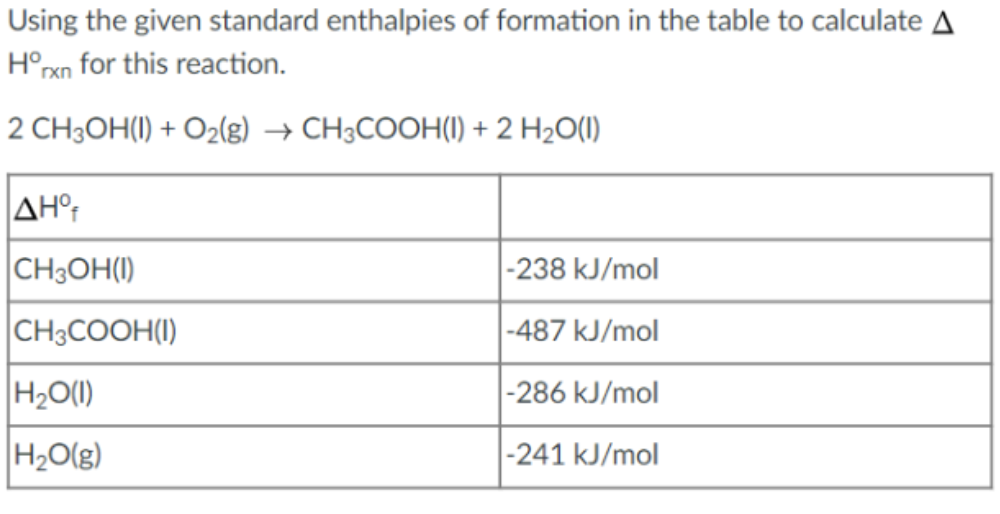
Transcribed Image Text:Using the given standard enthalpies of formation in the table to calculate A
Horxn for this reaction.
2 CH3OH(1) + O2(g) → CH3COOH(I) + 2 H2O(1)
AHof
CH3OH(1)
-238 kJ/mol
CH3COOH(1)
-487 kJ/mol
H2O(1)
-286 kJ/mol
H₂O(g)
-241 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning