value: 0.80 points 15 attempts left Check my work Be sure to answer all parts. At 1000 K, a sample of pure NO2 gas decomposes: 2N02(g) 2NO(g) + O2(g) The equilibrium constant, Kp, is 158. Analysis shows that the partial pressure of O2 is 0.17 atm at equilibrium. Calculate the pressure of NO and NO2 in the mixture. Pressure of NO: □atm Pressure of NO2: atm Type here to search Esc F1 F2 F3
value: 0.80 points 15 attempts left Check my work Be sure to answer all parts. At 1000 K, a sample of pure NO2 gas decomposes: 2N02(g) 2NO(g) + O2(g) The equilibrium constant, Kp, is 158. Analysis shows that the partial pressure of O2 is 0.17 atm at equilibrium. Calculate the pressure of NO and NO2 in the mixture. Pressure of NO: □atm Pressure of NO2: atm Type here to search Esc F1 F2 F3
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 76AP: Methanol can be synthesized by means of the equilibriumreaction CO(g)+2H2(g)CH3OH(g) for which the...
Related questions
Question
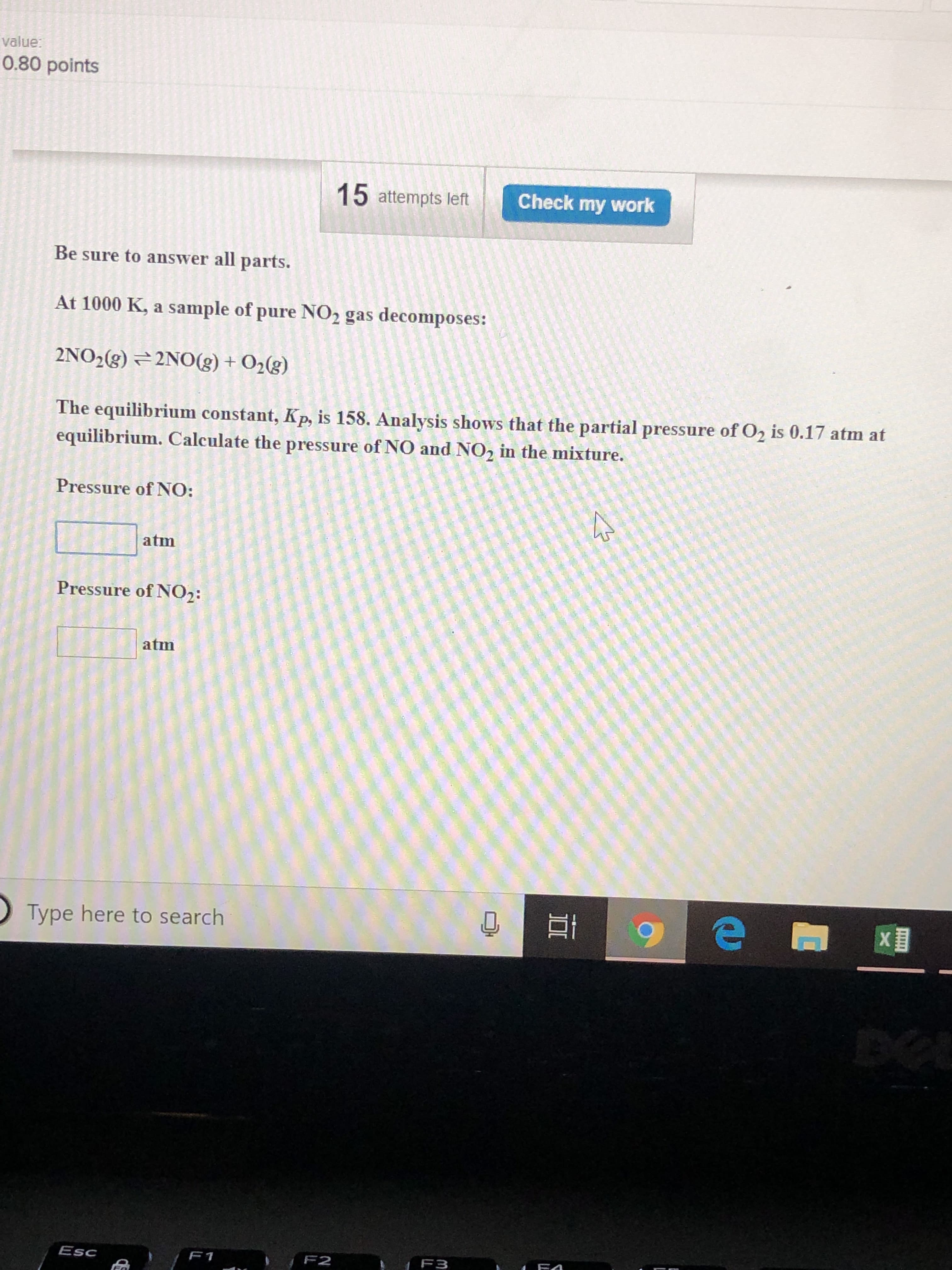
Transcribed Image Text:value:
0.80 points
15 attempts left
Check my work
Be sure to answer all parts.
At 1000 K, a sample of pure NO2 gas decomposes:
2N02(g) 2NO(g) + O2(g)
The equilibrium constant, Kp, is 158. Analysis shows that the partial pressure of O2 is 0.17 atm at
equilibrium. Calculate the pressure of NO and NO2 in the mixture.
Pressure of NO:
□atm
Pressure of NO2:
atm
Type here to search
Esc
F1
F2
F3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning