View Policies Current Attempt in Progress The pH of an NH4*/NH3 buffer solution is 9.04. Calculate the acid/conjugate base ratio for the solution. K for NH3 is 1.8E-5. i
View Policies Current Attempt in Progress The pH of an NH4*/NH3 buffer solution is 9.04. Calculate the acid/conjugate base ratio for the solution. K for NH3 is 1.8E-5. i
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.12QAP
Related questions
Question
100%
Transcribed Image Text:E Carleton 360 - Carleton Uni X
Homepage - Carleton Unive ×
NWP Assessment Builder UI App X
NWP Assessment Player UI Appl X
ny
O A https://education.wiley.com/was/ui/v2/assessment-player/index.html?launchld=3fbece73-937d-4463-a8ab-b80e42aaf607#/question/8
mment 6 (CH 16)
Question 9 of 25-
<>
-/1
View Policies
Current Attempt in Progress
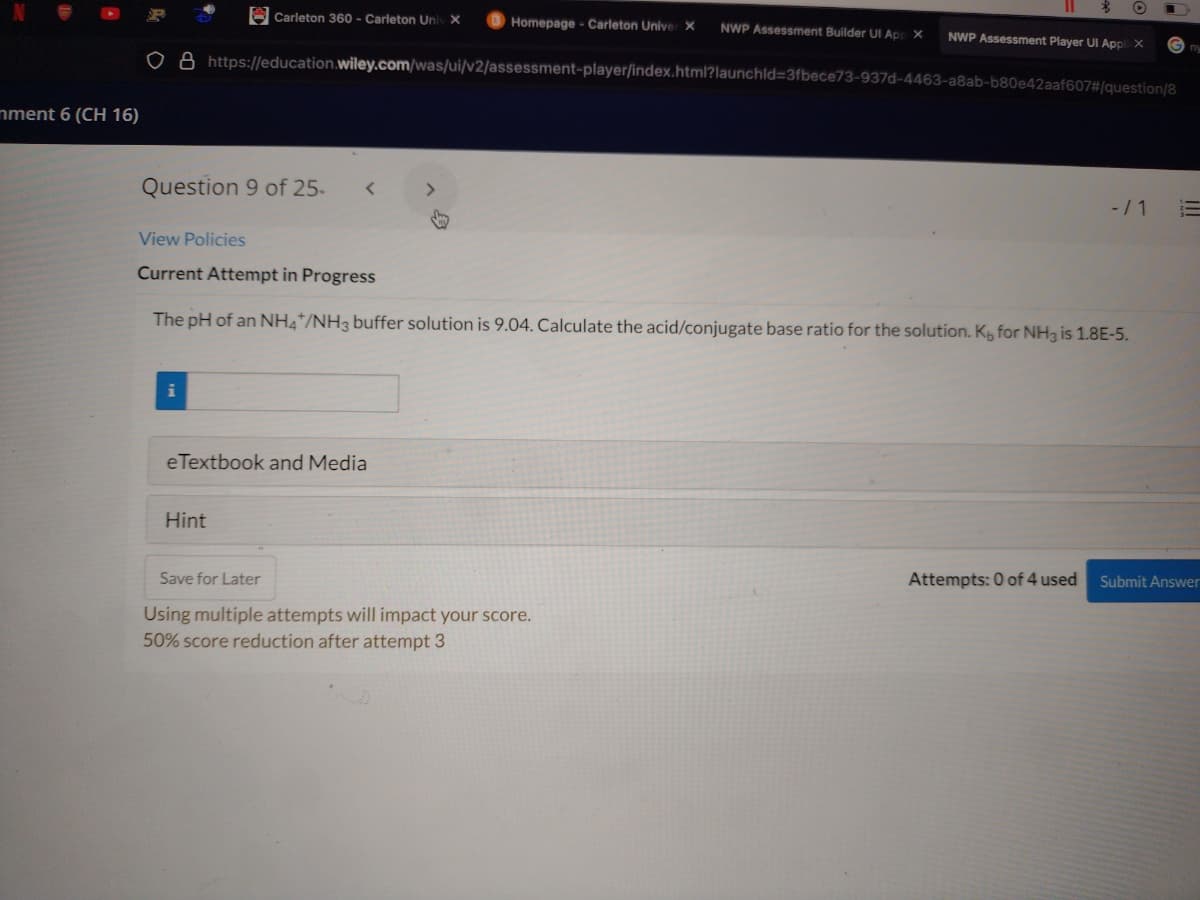
The pH of an NH4*/NH3 buffer solution is 9.04. Calculate the acid/conjugate base ratio for the solution. K for NH3 is 1.8E-5.
eTextbook and Media
Hint
Save for Later
Attempts: 0 of 4 used
Submit Answer
Using multiple attempts will impact your score.
50% score reduction after attempt 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning