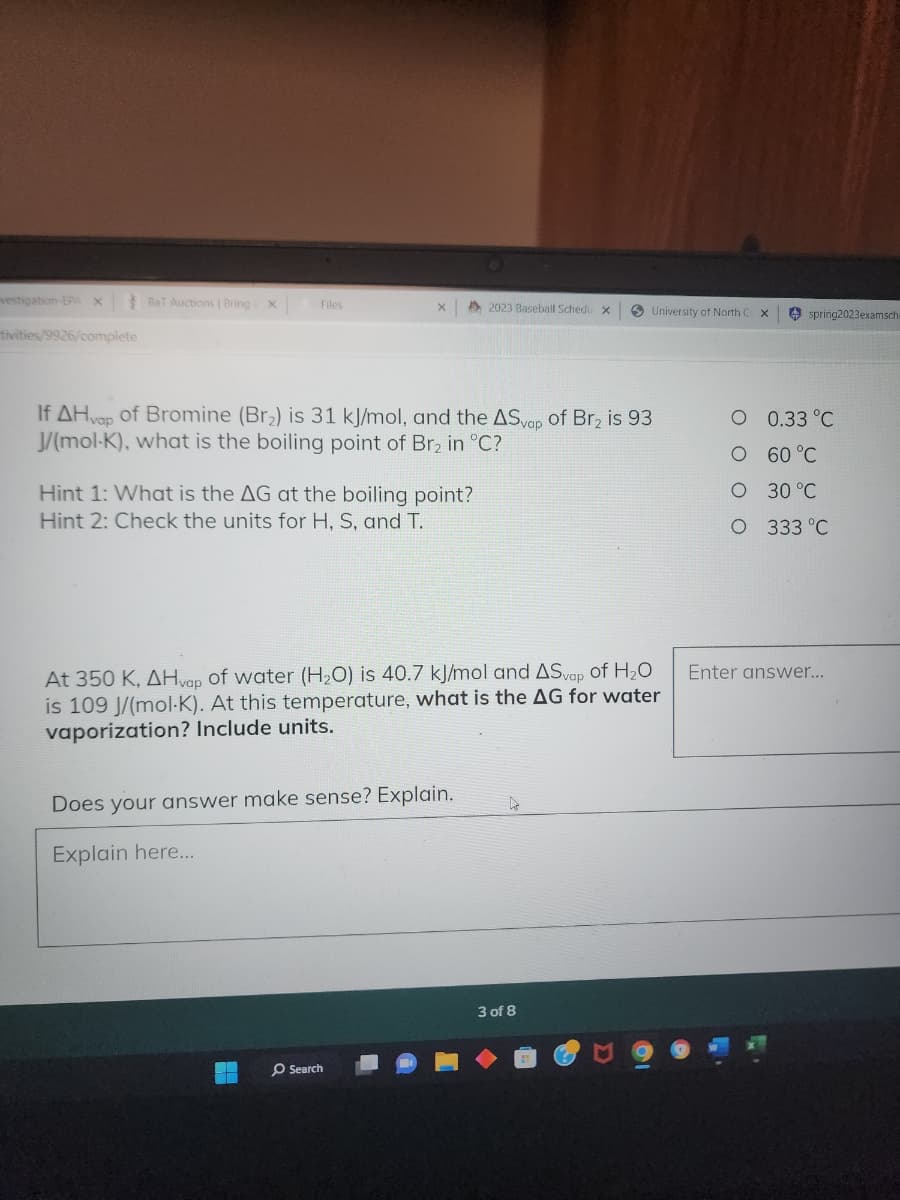
vities/9926/complete If AHvap of Bromine (Br₂) is 31 kJ/mol, and the ASvap of Br₂ is 93 J/(mol-K), what is the boiling point of Br₂ in °C? Hint 1: What is the AG at the boiling point? Hint 2: Check the units for H, S, and T. At 350 K, AHvap of water (H₂O) is 40.7 kJ/mol and ASvap of H₂O is 109 J/(mol-K). At this temperature, what is the AG for water vaporization? Include units. Does your answer make sense? Explain. Explain here... h O 0.33 °C O 60 °C O 30 °C O 333 °C Enter answer...
vities/9926/complete If AHvap of Bromine (Br₂) is 31 kJ/mol, and the ASvap of Br₂ is 93 J/(mol-K), what is the boiling point of Br₂ in °C? Hint 1: What is the AG at the boiling point? Hint 2: Check the units for H, S, and T. At 350 K, AHvap of water (H₂O) is 40.7 kJ/mol and ASvap of H₂O is 109 J/(mol-K). At this temperature, what is the AG for water vaporization? Include units. Does your answer make sense? Explain. Explain here... h O 0.33 °C O 60 °C O 30 °C O 333 °C Enter answer...
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 5RQ
Related questions
Question
Transcribed Image Text:vestigation-EPA X
tivities/9926/complete
BaT Auctions | Bring
Files
X
Hint 1: What is the AG at the boiling point?
Hint 2: Check the units for H, S, and T.
If AHvap of Bromine (Br₂) is 31 kJ/mol, and the ASvap of Br₂ is 93
J/(mol-K), what is the boiling point of Br₂ in °C?
2023 Baseball Schedu X
Does your answer make sense? Explain.
Explain here...
O Search
At 350 K, AHvap of water (H₂O) is 40.7 kJ/mol and ASvap of H₂O
is 109 J/(mol-K). At this temperature, what is the AG for water
vaporization? Include units.
University of North C X
3 of 8
O
O
O
spring2023examsche
0.33 °C
60 °C
30 °C
333 °C
Enter answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning