Volume Water 105.82 mL Initial Temperature of Water 25 °C Volume of Compound A 100 mL Initial Temperature of Compound A 60 °C Final temperature of Compound A + Water °C 25 Question 2 Calculate the density of Compound A. g/mL Question 3 Calculate the specific heat of Compound A from your data. The specific heat of wa is 4.184 J/g°C and its density is 1.00 g/mL. J/g°C
Volume Water 105.82 mL Initial Temperature of Water 25 °C Volume of Compound A 100 mL Initial Temperature of Compound A 60 °C Final temperature of Compound A + Water °C 25 Question 2 Calculate the density of Compound A. g/mL Question 3 Calculate the specific heat of Compound A from your data. The specific heat of wa is 4.184 J/g°C and its density is 1.00 g/mL. J/g°C
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.34PAE: 1.34 Convert the value 0.120 ppb into ppm.
Related questions
Question
Mass of Compound A 100 ML

Transcribed Image Text:250 mL Beaker
205.82 mL @ 25.0°C
Transcribed Image Text:Volume Water
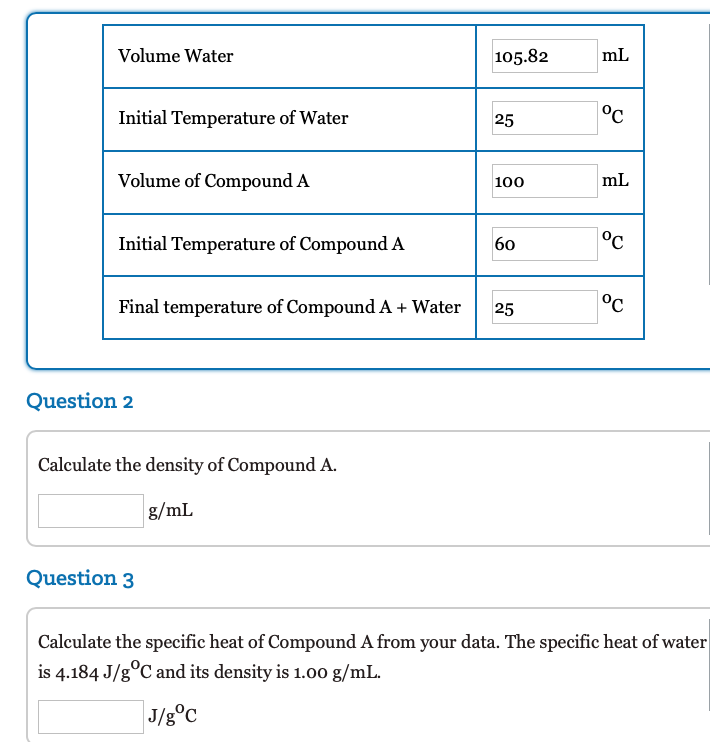
105.82
mL
Initial Temperature of Water
25
°c
Volume of Compound A
100
mL
Initial Temperature of Compound A
°C
60
Final temperature of Compound A + Water
25
°C
Question 2
Calculate the density of Compound A.
g/mL
Question 3
Calculate the specific heat of Compound A from your data. The specific heat of water
is 4.184 J/g°C and its density is 1.00 g/mL.
J/g°C
Expert Solution
Step 1
Ques 2) Mass of compound A=100 g
Calculation of the density of compound A:
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning