w officeapps.live.com/op/View.aspR? Download file and easy viewing right in Microsoft Edge. Choose Download file if you want to use it later. Lab 8 Answered v A Accessibility Mode I Download 4. Antacid tablets are used to neutralize stomach acid and relieve indigestion or acid reflux. Many commercial antacids contain magnesium hydroxide, Mg(OH)2, as the active ingredient. If one tablet of antacid contains 15 mg of Mg(OH)2, then calculate the number of tablets required to completely neutralize 5 mL of stomach acid if the stomach acid is 0.2 M HC1.
w officeapps.live.com/op/View.aspR? Download file and easy viewing right in Microsoft Edge. Choose Download file if you want to use it later. Lab 8 Answered v A Accessibility Mode I Download 4. Antacid tablets are used to neutralize stomach acid and relieve indigestion or acid reflux. Many commercial antacids contain magnesium hydroxide, Mg(OH)2, as the active ingredient. If one tablet of antacid contains 15 mg of Mg(OH)2, then calculate the number of tablets required to completely neutralize 5 mL of stomach acid if the stomach acid is 0.2 M HC1.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 109QRT: The grid has six lettered boxes, each of which contains an item that may be used to answer the...
Related questions
Question
100%
Show work
Transcribed Image Text:w.officeapps.live.com/op/VieW.aspx?STU
Download file
and easy viewing right in Microsoft Edge. Choose Download file if you want to use it later.
Lab 8 Answered v
A Accessibility Mode
I Download
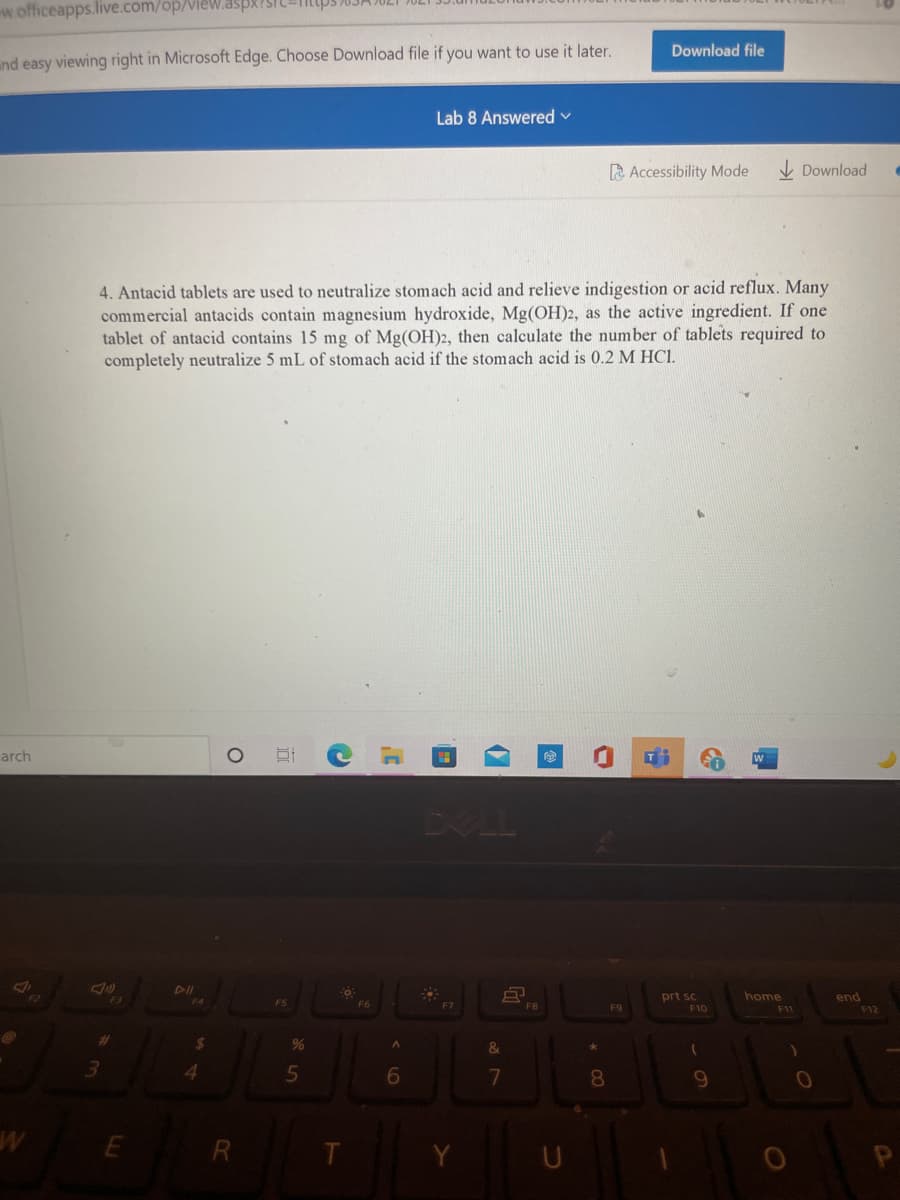
4. Antacid tablets are used to neutralize stomach acid and relieve indigestion or acid reflux. Many
commercial antacids contain magnesium hydroxide, Mg(OH)2, as the active ingredient. If one
tablet of antacid contains 15 mg of Mg(OH)2, then calculate the number of tablets required to
completely neutralize 5 mL of stomach acid if the stomach acid is 0.2 M HCI.
arch
DELL
DII
home
F11
prt sc
end
F5
F6
F7
F8
F9
F10
F12
%
9.
10
E R
T.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning