Water is exposed to infrared radiation with a wavelength of 3.0×10-4 cm . Assume that all the radiation is absorbed and converted to heat. Part A How many photons are required for the sample to absorb 13.55 J of heat? Express your answer using two significant figures. ? N =
Water is exposed to infrared radiation with a wavelength of 3.0×10-4 cm . Assume that all the radiation is absorbed and converted to heat. Part A How many photons are required for the sample to absorb 13.55 J of heat? Express your answer using two significant figures. ? N =
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter15: Introduction To Electronic Spectroscopy And Structure
Section: Chapter Questions
Problem 15.59E: Would the light from fireflies be considered an example of a fluorescence or a phosphorescence...
Related questions
Question
Please answer question 18 Part A
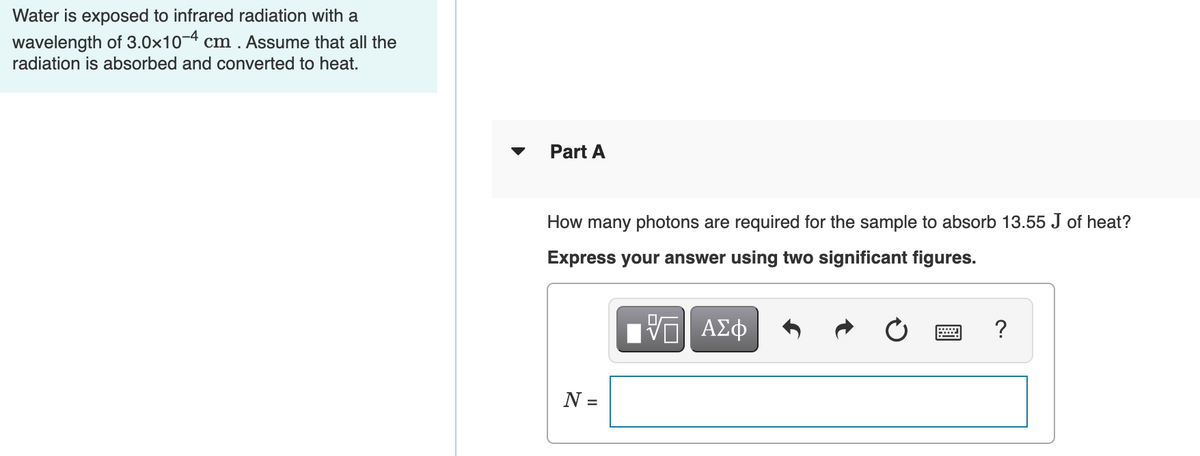
Transcribed Image Text:Water is exposed to infrared radiation with a
wavelength of 3.0x10-4 cm . Assume that all the
radiation is absorbed and converted to heat.
Part A
How many photons are required for the sample to absorb 13.55 J of heat?
Express your answer using two significant figures.
ΑΣφ
N =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning