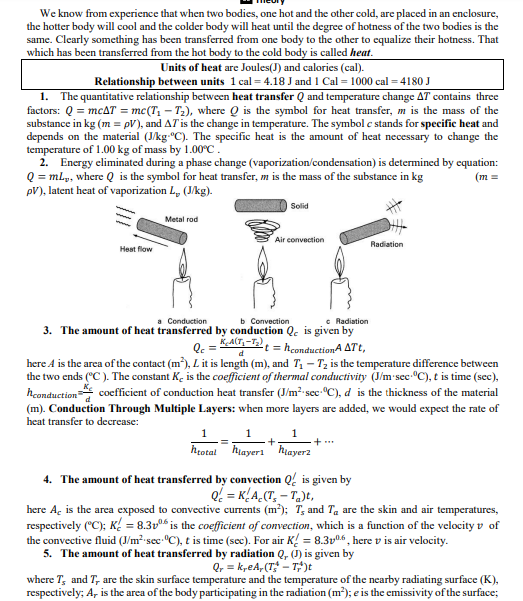
We know from experience that when two bodies, one hot and the other cold, are placed in an enclosure, the hotter body will cool and the colder body will heat until the degree of hotness of the two bodies is the same. Clearly something has been transferred from one body to the other to equalize their hotness. That which has been transferred from the hot body to the cold body is called heat. Units of heat are Joules(J) and calories (cal). Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J 1. The quantitative relationship between heat transfer Q and temperature change AT contains three factors: Q = mcAT = mc(T, – T2), where Q is the symbol for heat transfer, m is the mass of the substance in kg (m = pV), and AT is the change in temperature. The symbol c stands for specific heat and depends on the material (J/kg-"C). The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00°C. 2. Energy eliminated during a phase change (vaporization/condensation) is determined by equation: Q = ml,, where Q is the symbol for heat transfer, m is the mass of the substance in kg pV), latent heat of vaporization L, (Jkg). (m = Solid Metal rod Air convection Radiation Heat flow c Radiation 3. The amount of heat transferred by conduction Q. is given by = hconductionA ATt, a Conduction b Convection 0. - KACT,-T here A is the area of the contact (m), L it is length (m), and T, – Tz is the temperature difference between the two ends (°C ). The constant K. is the coefficient of thermal conductivity (J/m sec-°C), t is time (sec), hconduction coefficient of conduction heat transfer (J/m2-sec-C), d is the thickness of the material (m). Conduction Through Multiple Layers: when more layers are added, we would expect the rate of heat transfer to decrease: htotal hiayeri' hlayer2 4. The amount of heat transferred by convection Q is given by Q = K{A_(T, – T_)t, here A. is the area exposed to convective currents (m²); T, and Ta are the skin and air temperatures, respectively ("C); K! = 8.3vº6 is the coefficient of convection, which is a function of the velocity v of the convective fluid (J/m²•sec•°C), t is time (sec). For air K! = 8.3v0.6 , here v is air velocity. 5. The amount of heat transferred by radiation Q, () is given by Q, = k,eA, (T – T;*)t where T, and T, are the skin surface temperature and the temperature of the nearby radiating surface (K), respectively; A, is the area of the body participating in the radiation (m); e is the emissivity of the surface; and Kr is the radiation coefficient. Over a fairly wide range of temperatures, k, is, on the average, about k, = 5,67 · 10-8 J/(sec-m²-K*), t is time (sec), the emissivity of the skin e = 1. 6. The amount of heat that the human body receives from solar radiation is assuming that the full intensity of solar radiation reaches the surface Q = 150 eAcose Here A is the skin area of the person (m²), 0 is the angle of incidence of sunlight, and e is the emissivity of the skin. The emissivity of the skin in the wavelength region of solar radiation depends on the pigmentation. Dark skin absorbs about 80% of the radiation, and light skin absorbs about 60%. 6. The rate of the heat transfer W =! PROBLEMS 2. Find amount of heat removed from skin surface for each liter of sweat that evaporates from the skin. At normal skin temperatures (37° C) the latent heat of vaporization of water is L, = 2.4 x 106 J/kg and density of water p = 998.2 kg/m?.
We know from experience that when two bodies, one hot and the other cold, are placed in an enclosure, the hotter body will cool and the colder body will heat until the degree of hotness of the two bodies is the same. Clearly something has been transferred from one body to the other to equalize their hotness. That which has been transferred from the hot body to the cold body is called heat. Units of heat are Joules(J) and calories (cal). Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J 1. The quantitative relationship between heat transfer Q and temperature change AT contains three factors: Q = mcAT = mc(T, – T2), where Q is the symbol for heat transfer, m is the mass of the substance in kg (m = pV), and AT is the change in temperature. The symbol c stands for specific heat and depends on the material (J/kg-"C). The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00°C. 2. Energy eliminated during a phase change (vaporization/condensation) is determined by equation: Q = ml,, where Q is the symbol for heat transfer, m is the mass of the substance in kg pV), latent heat of vaporization L, (Jkg). (m = Solid Metal rod Air convection Radiation Heat flow c Radiation 3. The amount of heat transferred by conduction Q. is given by = hconductionA ATt, a Conduction b Convection 0. - KACT,-T here A is the area of the contact (m), L it is length (m), and T, – Tz is the temperature difference between the two ends (°C ). The constant K. is the coefficient of thermal conductivity (J/m sec-°C), t is time (sec), hconduction coefficient of conduction heat transfer (J/m2-sec-C), d is the thickness of the material (m). Conduction Through Multiple Layers: when more layers are added, we would expect the rate of heat transfer to decrease: htotal hiayeri' hlayer2 4. The amount of heat transferred by convection Q is given by Q = K{A_(T, – T_)t, here A. is the area exposed to convective currents (m²); T, and Ta are the skin and air temperatures, respectively ("C); K! = 8.3vº6 is the coefficient of convection, which is a function of the velocity v of the convective fluid (J/m²•sec•°C), t is time (sec). For air K! = 8.3v0.6 , here v is air velocity. 5. The amount of heat transferred by radiation Q, () is given by Q, = k,eA, (T – T;*)t where T, and T, are the skin surface temperature and the temperature of the nearby radiating surface (K), respectively; A, is the area of the body participating in the radiation (m); e is the emissivity of the surface; and Kr is the radiation coefficient. Over a fairly wide range of temperatures, k, is, on the average, about k, = 5,67 · 10-8 J/(sec-m²-K*), t is time (sec), the emissivity of the skin e = 1. 6. The amount of heat that the human body receives from solar radiation is assuming that the full intensity of solar radiation reaches the surface Q = 150 eAcose Here A is the skin area of the person (m²), 0 is the angle of incidence of sunlight, and e is the emissivity of the skin. The emissivity of the skin in the wavelength region of solar radiation depends on the pigmentation. Dark skin absorbs about 80% of the radiation, and light skin absorbs about 60%. 6. The rate of the heat transfer W =! PROBLEMS 2. Find amount of heat removed from skin surface for each liter of sweat that evaporates from the skin. At normal skin temperatures (37° C) the latent heat of vaporization of water is L, = 2.4 x 106 J/kg and density of water p = 998.2 kg/m?.
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 100P: One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a...
Related questions
Question
Find amount of heat removed from skin surface for each liter of sweat that evaporates from the
skin. At normal skin temperatures (370 C) the latent heat of vaporization of water is ?? = 2.4 × 106
J/kg
and density of water ? = 998.2 kg/m3
.
Transcribed Image Text:We know from experience that when two bodies, one hot and the other cold, are placed in an enclosure,
the hotter body will cool and the colder body will heat until the degree of hotness of the two bodies is the
same. Clearly something has been transferred from one body to the other to equalize their hotness. That
which has been transferred from the hot body to the cold body is called heat.
Units of heat are Joules(J) and calories (cal).
Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J
1. The quantitative relationship between heat transfer Q and temperature change AT contains three
factors: Q = mcAT = mc(T, – T2), where Q is the symbol for heat transfer, m is the mass of the
substance in kg (m = pV), and AT is the change in temperature. The symbol c stands for specific heat and
depends on the material (J/kg-"C). The specific heat is the amount of heat necessary to change the
temperature of 1.00 kg of mass by 1.00°C.
2. Energy eliminated during a phase change (vaporization/condensation) is determined by equation:
Q = ml,, where Q is the symbol for heat transfer, m is the mass of the substance in kg
pV), latent heat of vaporization L, (Jkg).
(m =
Solid
Metal rod
Air convection
Radiation
Heat flow
c Radiation
3. The amount of heat transferred by conduction Q. is given by
= hconductionA ATt,
a Conduction
b Convection
0. - KACT,-T
here A is the area of the contact (m), L it is length (m), and T, – Tz is the temperature difference between
the two ends (°C ). The constant K. is the coefficient of thermal conductivity (J/m sec-°C), t is time (sec),
hconduction coefficient of conduction heat transfer (J/m2-sec-C), d is the thickness of the material
(m). Conduction Through Multiple Layers: when more layers are added, we would expect the rate of
heat transfer to decrease:
htotal hiayeri' hlayer2
4. The amount of heat transferred by convection Q is given by
Q = K{A_(T, – T_)t,
here A. is the area exposed to convective currents (m²); T, and Ta are the skin and air temperatures,
respectively ("C); K! = 8.3vº6 is the coefficient of convection, which is a function of the velocity v of
the convective fluid (J/m²•sec•°C), t is time (sec). For air K! = 8.3v0.6 , here v is air velocity.
5. The amount of heat transferred by radiation Q, () is given by
Q, = k,eA, (T – T;*)t
where T, and T, are the skin surface temperature and the temperature of the nearby radiating surface (K),
respectively; A, is the area of the body participating in the radiation (m); e is the emissivity of the surface;
Transcribed Image Text:and Kr is the radiation coefficient. Over a fairly wide range of temperatures, k, is, on the average, about
k, = 5,67 · 10-8 J/(sec-m²-K*), t is time (sec), the emissivity of the skin e = 1.
6. The amount of heat that the human body receives from solar radiation is assuming that the full
intensity of solar radiation reaches the surface Q = 150 eAcose
Here A is the skin area of the person (m²), 0 is the angle of incidence of sunlight, and e is the emissivity
of the skin. The emissivity of the skin in the wavelength region of solar radiation depends on the
pigmentation. Dark skin absorbs about 80% of the radiation, and light skin absorbs about 60%.
6. The rate of the heat transfer W =!
PROBLEMS
2. Find amount of heat removed from skin surface for each liter of sweat that evaporates from the
skin. At normal skin temperatures (37° C) the latent heat of vaporization of water is L, = 2.4 x 106 J/kg
and density of water p = 998.2 kg/m?.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning