What biomolecule contains the genetic information of SARS-CoV-2? Be specific. -Describe the structure of the SARS-CoV-2. -What are the four main structural proteins in SARS-CoV-2? Briefly explain what each protein does. -How does SARS-CoV-2 bind to the host cell? Provide some evidences to support your answer. -What proteins can be targeted for therapeutic purposes (i.e. vaccines or antiviral drugs)?
What biomolecule contains the genetic information of SARS-CoV-2? Be specific. -Describe the structure of the SARS-CoV-2. -What are the four main structural proteins in SARS-CoV-2? Briefly explain what each protein does. -How does SARS-CoV-2 bind to the host cell? Provide some evidences to support your answer. -What proteins can be targeted for therapeutic purposes (i.e. vaccines or antiviral drugs)?
Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter21: Viruses
Section: Chapter Questions
Problem 19RQ: A patient presents at the clinic with an acute viral infection. Assays that analyze the viral life...
Related questions
Question
Read Section 3 to 3.5 and answer the ff:
-What biomolecule contains the genetic information of SARS-CoV-2? Be specific.
-Describe the structure of the SARS-CoV-2.
-What are the four main structural proteins in SARS-CoV-2? Briefly explain what each protein does.
-How does SARS-CoV-2 bind to the host cell? Provide some evidences to support your answer.
-What proteins can be targeted for therapeutic purposes (i.e. vaccines or antiviral drugs)?

Transcribed Image Text:G. Galer, H. Özdemir, D. Omar et al
information, Coronaviruses have infectious, non-segmented and a
single positive-sense RNA molecule. Coronaviruses and Toroviruses
are within the virus family, Coronavidiae. While coronaviruses have
pathogenic capacity on both animals and humans, Toroviruses are
one of the main reasons for animal diarrhea (Peiris, 2012), Coro-
naviruses can be divided into 4 groups known as alpha, beta, gamma
and delta, and people commonly get infected by alpha and beta
coronaviruses as known as: 229 E, NL63, OC43, and HKU1 (CDC.
2020). They are well known for their positive single-stranded
RNA genomes of approximately 30 kb, enveloped with nucleo-
capsid (Perlman and Netland, 2009). Recent studies have also
shown that current coronavirus threatening public health belongs
to betacoronavirus (Huang et al., 2020). It is really important to
understand the nature of a virus to come up with the right treat-
ment, thus, the studies regarding its pathogenesis carry a valid role.
To find a relation between SARS-CoV-2 and other coronaviruses, a
study calculated the sequence identity by collecting samples from
different types. It was revealed that samples from COVID-19
infected patients presented a close relationship with two bat-
derived coronaviruses: bat-SL-COVZC45 and bat-SL-CoVZXC21. In
fact, SARS-CoV-2 showed 88% identity with the two bat-derived
coronaviruses. In comparison to these viruses, the associations
between SARS-CoV-2 and SARS-CoV as well as MERS-CoV were
more distant. While SARS-CoV showed -79% identity with SARS-
CoV-2, MERS-CoV showed only -50% identity with SARS-CoV-2
(Lu et al., 2020). It was also reported that SARS-CoV-2 is 96%
identical at the whole-genome level to another bat coronavirus.
(BatCoV-RaTG13) (Zhou et al., 2020). This close relationship may
provide an evidence for SARS-CoV-2 to be originated in bats.
With the spread of the virus, new variants and mutations have
started to become a problem in some regions. In 2020 December, a
new variant appeared in the UK; it was revealed that this new
variant was associated with the spike protein of the virus. In late
December, it caused increased number of cases in the southeast of
England. Authorities stated that the new mutation would not cause
a major problem within the vaccine, as the vaccine affects many
regions in the spike protein (Wise, 2020). A matched cohort study
revealed the mortality rate to be slightly higher in the new strain
(Challen et al., 2021). Another variant named 501Y.V2 was found in
South Africa on December 18, 2020. It was discovered that this new
variant had spread to various provinces. Furthermore, this variant
was found to have 3 mutations in the spike protein (Tang et al.,
ang et
2021: "WHO | SARS-CoV-2 Variants," n. d.). Although the research
is still ongoing, according to a study, South African strain may be
more transmissible (Tegally et al., 2020),
Coronavirus has one of the best studied genome structures so
far, and exhibits a high mutation rate (Cui et al, 2019). Mutations in
this novel virus significantly affected the chain of transmission.
Although SARS-CoV-2 contains amino acid mutations in its
receptor-binding domain (Lu et al., 2020), both SARS-CoV and
SARS-CoV-2 use their spike proteins to recognize the angiotensin
converting enzyme 2 (ACE2) as a functional receptor on the target
cell for entry (Hoffmann et al., 2020; Lan et al., 2020; Letko et al..
2020; Shang et al., 2020; Walls et al., 2020; Wang et al., 2020b;
Yan et al., 2020). Recently, biophysical, and biochemical properties
of SARS-CoV-2 have been studied in detail, which will be addressed
in the following Sections 3 and 4.
3. Structural and functional features of SARS-CoV-2
Phylogenetic analyses revealed that SARS-CoV-2 belongs to the
betacoronavirus category of the Coronaviridae family. The corona-
virus disease 2019 (COVID-19), rapidly spreading worldwide, is
caused by this novel RNA virus SARS-CoV-2. To fight this global
crisis, effective treatment and vaccine alternatives against COVID-
Progress in Biophysics and Molecular Biology 164 (2021) 3-18
19 are urgently required. Obviously, understanding the structural
and functional features, dynamics and receptor recognition
mechanism of SARS-CoV-2 is the first crucial step to develop the
effective antiviral drug agents, novel targeted treatments, small
molecular inhibitors, blocking antibodies, other therapeutics as
well as to design an effective preventive vaccine against COVID-19.
Knowing the structures and interactions on the atomic level will
guide to identify potential targets by applying, for instance, in silico
structure-assisted drug design or molecular docking procedures to
find new drugs or to repurpose already approved drugs (Estrada,
2020; Hoffmann et al., 2020; Jin et al., 2020b). Hence, so far
structural and functional relationship of SARS-CoV-2 proteins have
been extensively studied by using biophysical techniques (e.g.
cryogenic electron microscopy (Cryo-EM), X-ray crystallography),
along with biochemical and biological assays (Lan et al., 2020;
Shang et al., 2020; Walls et al., 2020; Wang et al., 2020b; Wrapp
et al., 2020; Yan et al., 2020). Further studies on SARS-CoV-2 are
still ongoing with increasing tendency to have integrated knowl-
edge about molecular basis of recognition, molecular and atomic
interactions as well as conformational changes occurring during
viral attachment and entering into human cells.
Recently, biophysical and biochemical techniques have been
applied for characterization of SARS-CoV-2 in the COVID-19 studies.
Importantly, Cryo-EM (using deflection of electrons from frozen
protein samples at liquid nitrogen temperatures) and X-ray crys-
tallography (using scattering of X-rays from well-ordered protein
crystals) have been used to determine the 3D structure of SARS-
CoV-2 proteins at atomic resolution in a few Angstrom (A) range.
Later on, the atomic coordinates and maps of the protein 3D
structures have been deposited in the Protein Data Bank (PDB),
freely available to global community. In this regard, Lan et al.
determined the crystal structure of the receptor-binding domain
(RBD) of SARS-CoV-2 spike protein bound to the cell receptor ACE2
at 2.45 A resolution (PDB ID: 6M0J) by using X-ray diffraction data
(Lan et al., 2020). They also identified the amino acid residues of the
SARS-CoV-2 RBD, essential for the ACE2 binding. Walls et al.
determined the cryo-EM structures of the SARS-CoV-2 S ectodo-
main trimer in the closed (2.8 A resolution, PDB ID: 6VXX) and
partially opened (3.2 A resolution, PDB ID: 6VYB) conformations
(Walls et al., 2020). In another work, Wrapp et al. determined the
cryo-EM structure of the SARS-CoV-2 spike protein in the prefusion
conformation at 3.46 A resolution and identified that one of the
three receptor-binding domains is 'up' in a receptor-accessible
conformation (PDB ID: 6VSB) (Wrapp et al., 2020). They also
showed that SARS-CoV-2 S protein binds to human ACE2 with high
affinity by utilizing the surface plasmon resonance, which is an
optical technique used to determine the molecular interactions and
binding kinetics. Obtaining a large amount of SARS-CoV-2 proteins
(e.g. the spike protein) at high-quality is significant for develop-
ment of vaccine alternatives or for research purposes. In this
respect, Herrera et al. reported that ExpiCHO-S cell lines provided
enhanced yields of spike proteins (Herrera et al., 2020). To validate
the protein quality, stability and antigenicity, they analyzed the
expressed and purified spike proteins by using the biochemical,
biophysical and structural techniques and assays such as cryo-EM
Physica
(3D structure, protein conformation), enzyme-linked immunosor-
bent assay (ELISA; antigenicity), protein microarray (antigenicity),
flow cytometry (binding, specificity), analytical ultracentrifugation
(oligomerization), SDS-page (protein size, molecular mass), light
scattering (molecular mass, aggregation), and differential scanning
fluorimetry (thermal stability, melting temperature). In another
study associated with the COVID-19 studies, biochemical and bio-
physical characterization of the main protease, 3-chymotrypsin-
like protease (3CLpro) from SARS-CoV-2 has been recently reported
(Ferreira and Rabeh, 2020). Since 3CLpro of SARS-CoV-2 might be a
G. Güler, H. Özdemir, D. Omar et al.
promising antiviral drug target, its thermodynamic and kinetic
stability at various pH and salt buffering conditions were studied by
using differential scanning calorimetry, differential scanning fluo-
rimetry and circular dichroism (CD) spectroscopy. The far UV-CD
spectroscopic data in the 200-280 nm range was used for
tracking of secondary structural elements (eg. z-helix. B-sheets)
and thus protein structural integrity at various pH and salt buff-
ering conditions of 3CLpro. They reported that the protease has
relatively high thermodynamic stabilities over a wide pH range but
is less stable in the presence of salts. It is obvious that biophysical,
biochemical, structural, and dynamical characterization of SARS-
CoV-2 proteins helps to understand the viral features further, and
thus, providing enormous information to discover strategies to
combat COVID-19.
3.1. Structure of SARS-CoV-2
An RNA virus SARS-CoV-2 exhibits a spherical shape with a
diameter of 60-140 nm and has many spikes (9-12 nm long) on its
surface, and thus, it appears like a solar corona revealed by the
electron microscopic data (Zhu et al., 2020), SARS-CoV-2 contains a
positive-sense,single-stranded RNA genome. Its genome comprises
-29.8 kilobases-long RNA and has 11 open reading frames (ORFs),
including ORF1ab, ORF2, ORF3a, ORF4, ORFS, ORF6, ORF7a, ORF7b,
ORFS, ORF9 and ORF10 genes (Yoshimoto, 2020) (Fig. 1A). Accord-
ingly, ORF1ab gene expresses a large polyprotein which is further
cleaved by proteases into 16 nonstructural proteins (NSPs).
including also major enzymes like NSP3 (papain-like proteases,
PLP), NSPS (chymotrypsin-like protease, 3CLP), NSP12 (RNA-
dependent RNA polymerase, RdRp) and NSP13 (helicase, Hel) (Luk
et al., 2019; Yoshimoto, 2020), SARS-CoV-2 is made up of four main
structural proteins, responsible for replication processes and cell
entering. These proteins are the spike (S) protein encoded by ORF2,
the envelope (E) protein encoded by ORF4, the membrane (M)
protein encoded by ORFS, and the nucleocapsid (N) protein enco-
ded by ORF9. Besides, the gene fragments distributed among the
structural genes encodes the accessory/helper proteins (Lu et al.,
2020; Schoeman and Fielding, 2019; Wu et al., 2020; Yoshimoto,
2020). The enveloped structure of SARS-CoV-2 is made up of a
bilayer lipids and proteins (S glycoprotein, E, M). A ribonucleo-
protein core, which consists of the N protein bound to RNA genome,
is located inside this viral envelope (Fig. 1B) (Naqvi et al, 2020;
Yoshimoto, 2020)
The smallest structural protein of SARS-CoV-2 is the E protein
which is an integral membrane protein found in the viral mem-
brane. It plays a number of key roles in the viral replication such as
envelope formation, viral assembly, and it functions as ion channels
A
GENOME
5
OFFT
51 Subu
ORFID
RAD
51-52
Junction
201
SEMI
S
52 Subunit
T
NTD CTD 85-485 FP-HR 2 IM IC
▬▬▬
$2
Progress in Biophysics and Molecular Biology 164 (2021) 3-18
and interacts with host cell proteins (Li et al., 2020a; Naqvi et al.,
2020; Yoshimoto, 2020). The most abundant structural protein is
the M protein which is an integral membrane protein that spans the
viral membrane. It interacts with other main viral proteins and has
significant role in the RNA packaging and viral assembly, shaping
the viral envelope (Naqvi et al., 2020; Tang et al., 2020; Yoshimoto,
2020), The N protein is a -54 kDa protein that binds to viral RNA
structurally and assembles into a helical ribonucleocapsid. A recent
publication reported that its N-terminal is the RNA-binding domain
while its C-terminal domain is responsible for homodimerization
(PDB ID: 6WZO), very similar to that of SARS-CoV and MERS-CoV.
and it forms large oligomers (tetramers) likely through nucleic
acid-protein and protein-protein interactions (Ye et al., 2020). This
nucleocapsid phosphoprotein is involved in the transcription,
replication and packaging of the viral RNA (Naqvi et al., 2020;
Schoeman and Fielding, 2019; Ye et al., 2020; Yoshimoto, 2020), The
spike (S) proteins coated with polysaccharides are glycoproteins
that a large number of S proteins protrude like a pedal-shaped
spike on the surface of the virus envelope (Fig. 2A). The S protein
has 2 vital subunits for binding (S1 subunit) and fusion (S2 subunit)
with the host cell. Briefly, the S protein facilities the entry into the
human cells by attaching to a specific receptor (ACE2) located on
the surface of the host cell (Fig. 2B). Those main structural proteins
addressed above have collateral effects on the viral infection, and
thus, they can be considered as potential drug targets as well. In the
following sections of biophysical parts, the characteristics of spike
protein can be comprehended in a more detailed way, which can
help with the understanding of the connection of the virus with the
human cells.
3.2. Structure of spike (S) protein
2017
Currently, it is well known that the COVID-19 infection initiates
with the interaction of SARS-CoV-2 spike (S) protein (termed SARS-
CoV-2 S) with human cells as a first step (Fig. 2B). The S proteins of
both SARS-CoV-2 and SARS-CoV bind to human ACE2 receptor at
high affinities in the nanomolar range to enter and invade the
target human cells (Walls et al., 2020; Wang et al., 2020b; Wrapp
et al., 2020). Thus, SARS-CoV-2 S protein is an essential target for
therapeutic, diagnostic and biological research.
SARS-CoV-2 S protein has a high amino acid sequence identity
with the S glycoproteins of SARS-CoV (76%) and BatCoV-RaTG13
(97%) (Walls et al, 2020; Yoshimoto, 2020; Zhou et al., 2020).
SARS-CoV-2 has a longer spike protein with a length of 1273 amino
acids in comparison to both SARS-CoV and MERS-CoV (Lu et al.,
2020). A recent cryo-EM structural data revealed that SARS-CoV-2
S protein has a total structural weight of -438 kDa (Pdb ID:
B
Protein (M)
RNA-
A
Spike Protein (5)
theo th
Protein (N)
Envelope Protein (E)
Fig. 1. Schematic representations of SARS-CoV-2 genome and structure. (A) Single-stranded RNA genome with a length of -29.8 kb, showing the spike protein in detail. (B) Structure
of SARS-CoV-2, demonstrating its main structural proteins which are spike protein, membrane protein, envelope protein, and nucleocapsid protein. (Created with BioRender
Transcribed Image Text:G. Güler, H. Ozdemir, D. Omar et al.
A
RBM receptor binding
52 sub (fin)
51 subunit (BD
receptor-binding
domain)
Viral
membrane
eruscellular tall
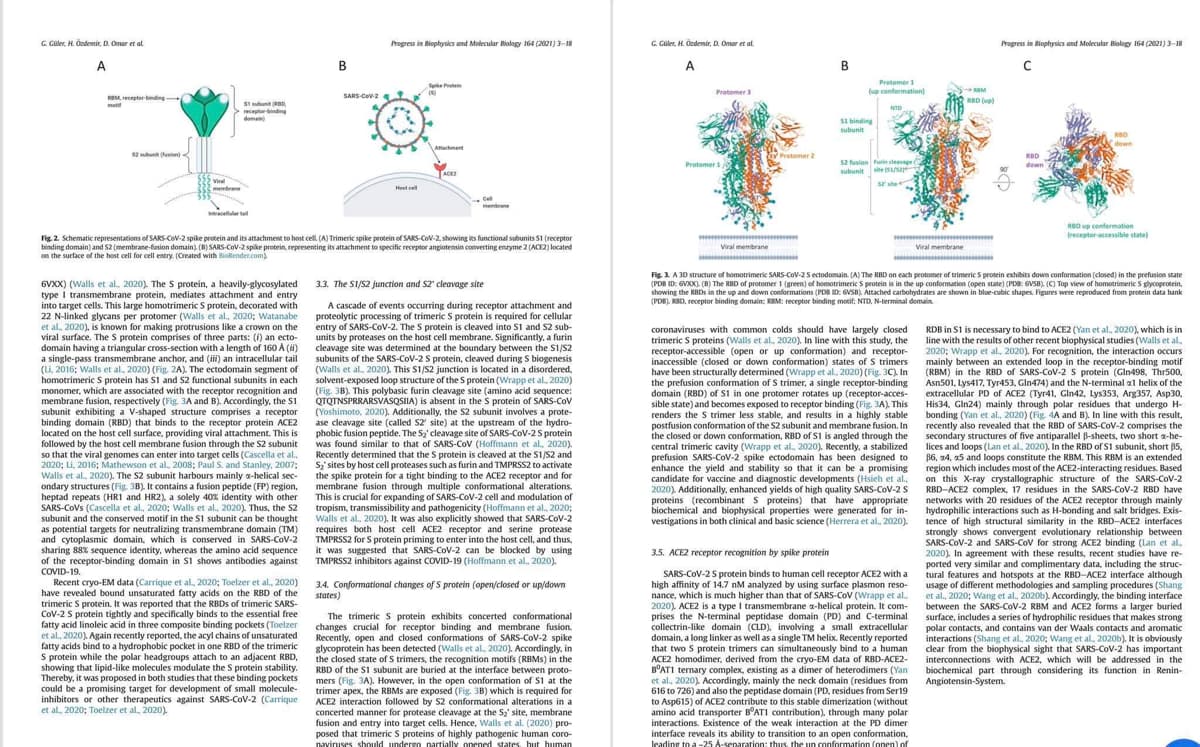
6VXX) (Walls et al., 2020). The S protein, a heavily-glycosylated
type I transmembrane protein, mediates attachment and entry
into target cells. This large homotrimeric S protein, decorated with
22 N-linked glycans per protomer (Walls et al., 2020; Watanabe
et al., 2020), is known for making protrusions like a crown on the
viral surface. The S protein comprises of three parts: (f) an ecto-
domain having a triangular cross-section with a length of 160 A (ii)
a single-pass transmembrane anchor, and (iii) an intracellular tail
(Li, 2016; Walls et al., 2020) (Fig. 2A). The ectodomain segment of
homotrimeric S protein has S1 and S2 functional subunits in each
monomer, which are associated with the receptor recognition and
membrane fusion, respectively (Fig. 3A and B). Accordingly, the S1
subunit exhibiting a V-shaped structure comprises a receptor
binding domain (RBD) that binds to the receptor protein ACE2
located on the host cell surface, providing viral attachment. This is
followed by the host cell membrane fusion through the S2 subunit
so that the viral genomes can enter into target cells (Cascella et al.,
2020; Li, 2016; Mathewson et al., 2008; Paul S. and Stanley, 2007;
Walls et al., 2020). The S2 subunit harbours mainly a-helical sec-
ondary structures (Fig. 3B). It contains a fusion peptide (FP) region,
heptad repeats (HR1 and HR2), a solely 40% identity with other
SARS-CoVs (Cascella et al., 2020; Walls et al., 2020). Thus, the S2
subunit and the conserved motif in the S1 subunit can be thought
as potential targets for neutralizing transmembrane domain (TM)
and cytoplasmic domain, which is conserved in SARS-CoV-2
sharing 88% sequence identity, whereas the amino acid sequence
of the receptor-binding domain in S1 shows antibodies against
COVID-19.
B
Recent cryo-EM data (Carrique et al., 2020; Toelzer et al., 2020)
have revealed bound unsaturated fatty acids on the RBD of the
trimeric S protein. It was reported that the RBDs of trimeric SARS-
CoV-2 S protein tightly and specifically binds to the essential free
fatty acid linoleic acid in three composite binding pockets (Toelzer
et al., 2020). Again recently reported, the acyl chains of unsaturated
fatty acids bind to a hydrophobic pocket in one RBD of the trimeric
S protein while the polar headgroups attach to an adjacent RBD,
showing that lipid-like molecules modulate the S protein stability.
Thereby, it was proposed in both studies that these binding pockets
could be a promising target for development of small molecule-
inhibitors or other therapeutics against SARS-CoV-2 (Carrique
et al., 2020; Toelzer et al., 2020).
SARS-CoV-2
Progress in Biophysics and Molecular Biology 164 (2021) 3-18
Host cell
SP
(8)
Attachment
ACE2
Fig. 2. Schematic representations of SARS-CoV-2 spike protein and its attachment to host cell. (A) Trimeric spike protein of SARS-CoV-2, showing its functional subunits 51 (receptor
binding domain) and 52 (membrane-fusion domain). (B) SARS-CoV-2 spike protein, representing its attachment to specific receptor angiotensin converting enzyme 2 (ACE2) located
on the surface of the host cell for cell entry. (Created with BioRender.com)
Cell
membrane
3.3. The S1/S2 junction and S2' cleavage site
A cascade of events occurring during receptor attachment and
proteolytic processing of trimeric S protein is required for cellular
entry of SARS-CoV-2. The S protein is cleaved into S1 and S2 sub-
units by proteases on the host cell membrane. Significantly, a furin
cleavage site was determined at the boundary between the S1/S2
subunits of the SARS-CoV-2 S protein, cleaved during S biogenesis
(Walls et al., 2020). This S1/S2 junction is located in a disordered,
solvent-exposed loop structure of the S protein (Wrapp et al., 2020)
(Fig. 3B). This polybasic furin cleavage site (amino acid sequence:
QTQTNSPRRARSVASQSIIA) is absent in the S protein of SARS-CoV
(Yoshimoto, 2020). Additionally, the S2 subunit involves a prote-
ase cleavage site (called S2' site) at the upstream of the hydro-
phobic fusion peptide. The S₂' cleavage site of SARS-CoV-2 S protein
was found similar to that of SARS-CoV (Hoffmann et al., 2020).
Recently determined that the S protein is cleaved at the S1/S2 and
S₂' sites by host cell proteases such as furin and TMPRSS2 to activate
the spike protein for a tight binding to the ACE2 receptor and for
membrane fusion through multiple conformational alterations.
This is crucial for expanding of SARS-CoV-2 cell and modulation of
tropism, transmissibility and pathogenicity (Hoffmann et al., 2020;
Walls et al., 2020). It was also explicitly showed that SARS-CoV-2
requires both host cell ACE2 receptor and serine protease
TMPRSS2 for S protein priming to enter into the host cell, and thus,
it was suggested that SARS-CoV-2 can be blocked by using
TMPRSS2 inhibitors against COVID-19 (Hoffmann et al., 2020).
3.4. Conformational changes of S protein (open/closed or up/down
states)
The trimeric S protein exhibits concerted conformational
changes crucial for receptor binding and membrane fusion.
Recently, open and closed conformations of SARS-CoV-2 spike
glycoprotein has been detected (Walls et al., 2020). Accordingly, in
the closed state of S trimers, the recognition motifs (RBMs) in the
RBD of the S1 subunit are buried at the interface between proto-
mers (Fig. 3A). However, in the open conformation of S1 at the
trimer apex, the RBMs are exposed (Fig. 3B) which is required for
ACE2 interaction followed by S2 conformational alterations in a
concerted manner for protease cleavage at the S₂' site, membrane
fusion and entry into target cells. Hence, Walls et al. (2020) pro-
posed that trimeric S proteins of highly pathogenic human coro-
naviruses should undergo partially opened states, but human
G. Güler, H. Özdemir, D. Omar et al
A
Protomer 3
Protomer 1,
*******
Viral membrane
Protomer 2
B
Protomer 1
(up conformation)
$1 binding
subunit
NTD
52 fusion Furin cleavage
subunit
site (51/52
52 site
coronaviruses with common colds should have largely closed
trimeric S proteins (Walls et al., 2020). In line with this study, the
receptor-accessible (open or up conformation) and receptor-
inaccessible (closed or down conformation) states of S trimers
have been structurally determined (Wrapp et al., 2020) (Fig. 3C). In
the prefusion conformation of S trimer, a single receptor-binding
domain (RBD) of S1 in one protomer rotates up (receptor-acces-
sible state) and becomes exposed to receptor binding (Fig. 3A). This
renders the S trimer less stable, and results in a highly stable
postfusion conformation of the S2 subunit and membrane fusion. In
the closed or down conformation, RBD of S1 is angled through the
central trimeric cavity (Wrapp et al., 2020). Recently, a stabilized
prefusion SARS-CoV-2 spike ectodomain has been designed to
enhance the yield and stability so that it can be a promising
candidate for vaccine and diagnostic developments (Hsieh et al..
2020). Additionally, enhanced yields of high quality SARS-CoV-2 S
proteins (recombinant S proteins) that have appropriate
biochemical and biophysical properties were generated for in-
vestigations in both clinical and basic science (Herrera et al., 2020).
3.5. ACE2 receptor recognition by spike protein
SARS-CoV-2 S protein binds to human cell receptor ACE2 with a
high affinity of 14.7 nM analyzed by using surface plasmon reso-
nance, which is much higher than that of SARS-CoV (Wrapp et al..
2020), ACE2 is a type I transmembrane x-helical protein. It com-
prises the N-terminal peptidase domain (PD) and C-terminal.
collectrin-like domain (CLD), involving a small extracellular
domain, a long linker as well as a single TM helix. Recently reported
that two S protein trimers can simultaneously bind to a human
ACE2 homodimer, derived from the cryo-EM data of RBD-ACE2-
BOAT1 ternary complex, existing as a dimer of heterodimers (Yan
et al., 2020). Accordingly, mainly the neck domain (residues from
616 to 726) and also the peptidase domain (PD, residues from Ser19
to Asp615) of ACE2 contribute to this stable dimerization (without
amino acid transporter BOAT1 contribution), through many polar
interactions. Existence of the weak interaction at the PD dimer
wwwwww
interface reveals its ability to transition to an open conformation,
leading to a -25 A-separation: thus, the un conformation (open) of
REM
RBD (up)
********************
Viral membrane
***********
Progress in Biophysics and Molecular Biology 164 (2021) 3-18
C
RBD
down
RBD
Fig. 3. A 3D structure of homotrimeric SARS-CoV-2 S ectodomain. (A) The RBD on each protomer of trimeric S protein exhibits down conformation (closed) in the prefusion state
(PDB ID: 6VXX). (B) The RBD of protomer 1 (green) of homotrimeric S protein is in the up conformation (open state) (PDB: 6VSB). (C) Top view of homotrimeric S glycoprotein,
showing the RBDs in the up and down conformations (PDB ID: GVSB), Attached carbohydrates are shown in blue-cubic shapes. Figures were reproduced from protein data bank
(PDB), RBD, receptor binding domain; KBM: receptor binding motif; NTD, N-terminal domain
RBD up conformation
[receptor-accessible state)
RDB in S1 is necessary to bind to ACE2 (Yan et al., 2020), which is in
line with the results of other recent biophysical studies (Walls et al.,
2020; Wrapp et al., 2020). For recognition, the interaction occurs
mainly between an extended loop in the receptor-binding motif
(RBM) in the RBD of SARS-CoV-2 S protein (Gln498, Thr500,
Asn501, Lys417, Tyr453, Gln474) and the N-terminal 1 helix of the
extracellular PD of ACE2 (Tyr41, Gln42, Lys353, Arg357, Asp30.
His34, Gln24) mainly through polar residues that undergo H-
bonding (Yan et al., 2020) (Fig. 4A and B). In line with this result,
recently also revealed that the RBD of SARS-CoV-2 comprises the
secondary structures of five antiparallel ß-sheets, two short a-he-
lices and loops (Lan et al., 2020). In the RBD of S1 subunit, short 85,
86, 24, 25 and loops constitute the RBM. This RBM is an extended
region which includes most of the ACE2-interacting residues. Based
on this X-ray crystallographic structure of the SARS-CoV-2
RBD-ACE2 complex, 17 residues in the SARS-CoV-2 RBD have
networks with 20 residues of the ACE2 receptor through mainly
hydrophilic interactions such as H-bonding and salt bridges. Exis-
tence of high structural similarity in the RBD-ACE2 interfaces
strongly shows convergent evolutionary relationship between
SARS-CoV-2 and SARS-CoV for strong ACE2 binding (Lan et al.
2020). In agreement with these results, recent studies have re-
ported very similar and complimentary data, including the struc-
tural features and hotspots at the RBD-ACE2 interface although
usage of different methodologies and sampling procedures (Shang
et al., 2020; Wang et al., 2020b). Accordingly, the binding interface
between the SARS-CoV-2 RBM and ACE2 forms a larger buried
surface, includes a series of hydrophilic residues that makes strong
polar contacts, and contains van der Waals contacts and aromatic
interactions (Shang et al., 2020; Wang et al., 2020b). It is obviously
clear from the biophysical sight that SARS-CoV-2 has important
interconnections with ACE2, which will be addressed in the
biochemical part through considering its function in Renin-
Angiotensin-System.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning