What does the law of definite proportions state? Select the correct answer below: All samples of a pure compound contain the same elements in the same proportion by mass. When two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small, whole numbers. For every action there is an equal and opposite reaction. The total energy of the universe is constant. D FEEDBA
What does the law of definite proportions state? Select the correct answer below: All samples of a pure compound contain the same elements in the same proportion by mass. When two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small, whole numbers. For every action there is an equal and opposite reaction. The total energy of the universe is constant. D FEEDBA
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter3: Atomic Structures And The Periodic Table
Section: Chapter Questions
Problem 3.101EP
Related questions
Question
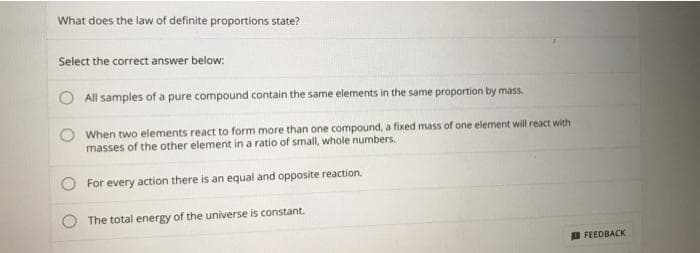
Transcribed Image Text:What does the law of definite proportions state?
Select the correct answer below:
All samples of a pure compound contain the same elements in the same proportion by mass.
When two elements react to form more than one compound, a fixed mass of one element will react with
masses of the other element in a ratio of small, whole numbers.
For every action there is an equal and opposite reaction.
The total energy of the universe is constant.
D FEEDBACK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning