Glencoe Physics: Principles and Problems, Student Edition
1st Edition
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Paul W. Zitzewitz
Chapter13: State Of Matter
Section: Chapter Questions
Problem 2STP
Related questions
Question
What is Relation between volume and pressure at constant temperature and number of moles.
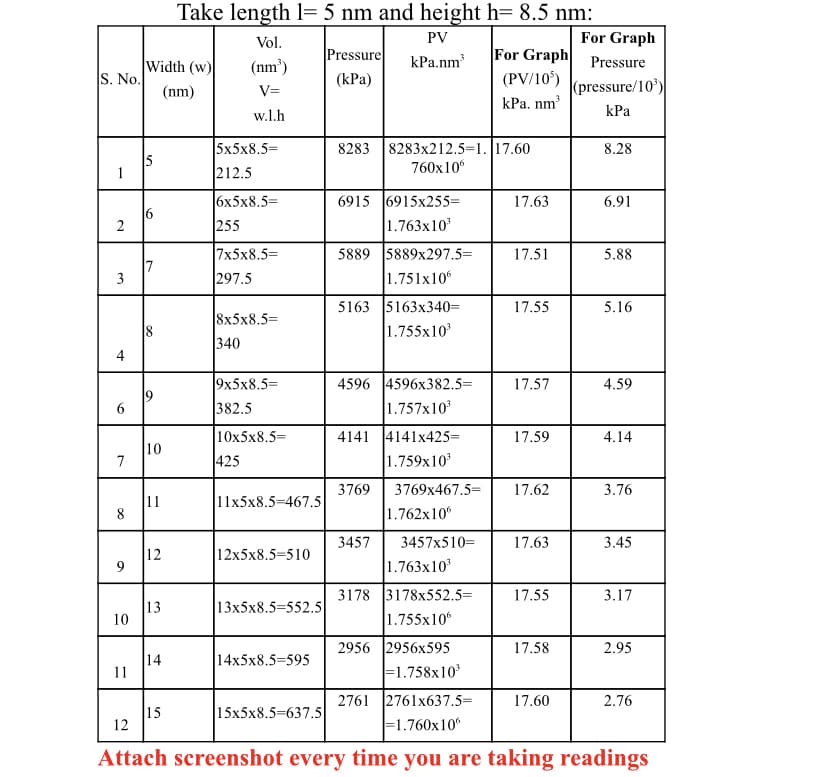
Transcribed Image Text:Take length l= 5 nm and height h= 8.5 nm:
For Graph
For Graph Pressure
Vol.
PV
Pressure
kPa.nm
Width (w)
S. No.
(nm')
(PV/10')
kPa. nm
(kPa)
(nm)
V=
(pressure/10')
w.l.h
КРа
5x5x8.5=
8283 |8283x212.5=1. 17.60
760x10
8.28
212.5
6915 6915x255=
1.763x10
6x5x8.5=
17.63
6.91
6
255
5889 5889x297.5=
7x5x8.5=
297.5
17.51
5.88
7
3
1.751x106
5163 5163x340=
1.755x10
17.55
5.16
8x5x8.5=
8
340
4
9x5x8.5=
382.5
4596 4596x382.5=
1.757x10
17.57
4.59
10x5x8.5%=
4141 4141x425=
1.759x10
17.59
4.14
10
7
425
3769
3769x467.5=
17.62
3.76
11
8
11x5x8.5-467.5
1.762x10
3457
3457x510=
17.63
3.45
12
9
12x5x8.5=510
1.763x10
3178 3178x552.5%=
1.755x10
17.55
3.17
13
10
13x5x8.5=552.5
2956 2956x595
F1.758x10
17.58
2.95
14
11
14x5x8.5=595
2761 2761x637.5=
17.60
2.76
15
12
|15x5x8.5-637.5
F1.760x10°
Attach screenshot every time you are taking readings
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College