What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu+ concentration is 1.07 M and the Al* concentration is 7.70 x 10 M? 3Cu" (aq) + 2AI(s) + 3Cu(s) + 2A1* (aq) Ecell =| The cell reaction as written above is for the concentrations given. spontaneous nonspontaneous www r pts remaining Submit Answer Retry Entire Group
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu+ concentration is 1.07 M and the Al* concentration is 7.70 x 10 M? 3Cu" (aq) + 2AI(s) + 3Cu(s) + 2A1* (aq) Ecell =| The cell reaction as written above is for the concentrations given. spontaneous nonspontaneous www r pts remaining Submit Answer Retry Entire Group
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 31E: Determine the standard cell potential and the cell potential under the stated conditions for the...
Related questions
Question
100%
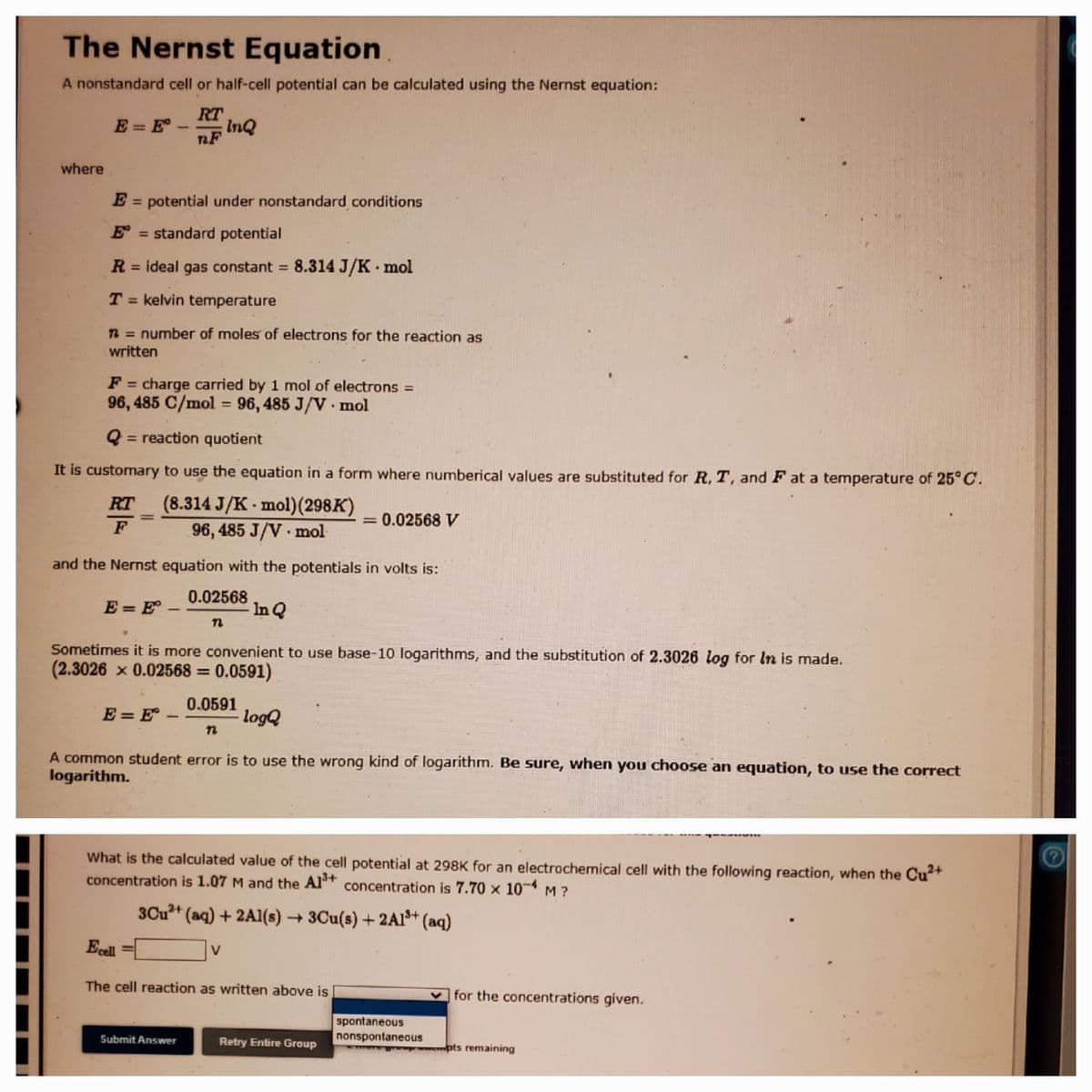
Transcribed Image Text:The Nernst Equation
A nonstandard cell or half-cell potential can be calculated using the Nernst equation:
RT
E = E-
InQ
nF
where
E = potential under nonstandard conditions
E = standard potential
R = ideal gas constant 8.314 J/K mol
%3D
%3D
T = kelvin temperature
n = number of moles of electrons for the reaction as
written
%3D
F = charge carried by 1 mol of electrons =
96, 485 C/mol = 96, 485 J/V · mol
%3D
%3D
= reaction quotient
It is customary to use the equation in a form where numberical values are substituted for R, T, and F at a temperature of 25°C.
RT (8.314 J/K - mol)(298K)
96, 485 J/V mol
= 0.02568 V
%3D
F
and the Nernst equation with the potentials in volts is:
E = E° –
0.02568
In Q
-
Sometimes it is more convenient to use base-10 logarithms, and the substitution of 2.3026 log for In is made.
(2.3026 x 0.02568 = 0.0591)
E = E° –
0.0591
logQ
A common student error is to use the wrong kind of logarithm. Be sure, when you choose an equation, to use the correct
logarithm.
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu+
concentration is 1.07 M and the Al* concentration is 7.70 × 10¬ M ?
3Cu+ (aq) + 2Al(s) → 3Cu(s) + 2Al* (aq)
Ecel
V
The cell reaction as written above is
v for the concentrations given.
spontaneous
nonspontaneous
Submit Answer
Retry Entire Group
pts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning