Data Table 1 No. of Metal Tested Mg2. (MgSO4) Fe2+ (FeSO4) Cu2+ (CUSO4) Reactions A lot of audible fizzing, a lot of tarnishing, color of CusOs changed from bright blue to a dark green/blue Pin turned a copper/orange Mg(s) Some bubbles, audible fizzing, slight tarnishing Nothing Fe(s) Nothing Slight tarnish 1- color change color Particle of copper in the Cu(s) nothing Slight tarnish solution Photo 1 Fe* Cu Mg Fe Cu
Data Table 1 No. of Metal Tested Mg2. (MgSO4) Fe2+ (FeSO4) Cu2+ (CUSO4) Reactions A lot of audible fizzing, a lot of tarnishing, color of CusOs changed from bright blue to a dark green/blue Pin turned a copper/orange Mg(s) Some bubbles, audible fizzing, slight tarnishing Nothing Fe(s) Nothing Slight tarnish 1- color change color Particle of copper in the Cu(s) nothing Slight tarnish solution Photo 1 Fe* Cu Mg Fe Cu
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section: Chapter Questions
Problem 57GQ: Hemoglobin (Hb) can form a complex with both O2 and CO. For the reaction HbO2(aq) + CO(g) HbCO(aq)...
Related questions
Question
What are the number of reactions based on the observations, sound is not a reaction right?
And, based on the data collected, list the metals you used in order from most reactive to least reactive. Explain your reasoning.
Thanks!
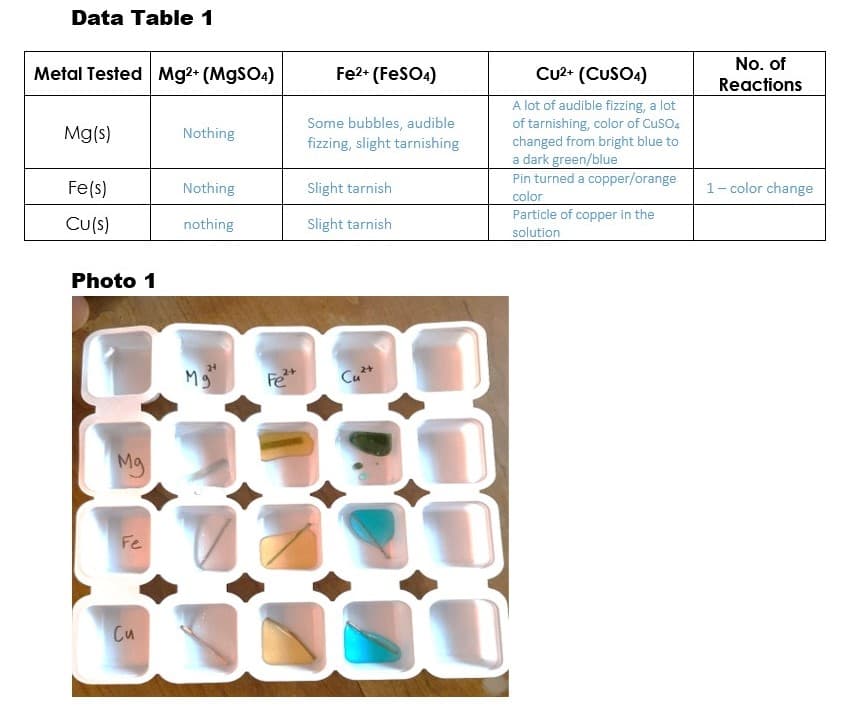
Transcribed Image Text:Data Table 1
No. of
Metal Tested Mg2+ (MgSO4)
Fe2+ (FeSO4)
CU2+ (CUSOA)
Reactions
A lot of audible fizzing, a lot
of tarnishing, color of CusO4
changed from bright blue to
a dark green/blue
Pin turned a copper/orange
Mg(s)
Some bubbles, audible
fizzing, slight tarnishing
Nothing
Fe(s)
Nothing
Slight tarnish
1- color change
color
Cu(s)
Particle of copper in the
solution
nothing
Slight tarnish
Photo 1
Fe*
Cu*+
Mg
Mg
Fe
Cu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


