What is the heat of reaction? Is the reaction exothermic or endothermic? Are the reactants (A + B) or the products (C+D) more chemically stable? Can you determine the activation energy? Can you determine the activation energy of the reverse reaction? C+DA+B kJ/mol Exothermic O Endothermic Neither Reactants (A + B) Products (C+D) Neither Yes, it's kJ/mol No. Yes, it's kJ/mol No.
What is the heat of reaction? Is the reaction exothermic or endothermic? Are the reactants (A + B) or the products (C+D) more chemically stable? Can you determine the activation energy? Can you determine the activation energy of the reverse reaction? C+DA+B kJ/mol Exothermic O Endothermic Neither Reactants (A + B) Products (C+D) Neither Yes, it's kJ/mol No. Yes, it's kJ/mol No.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.12: Over The Hill: Reversing Reactions
Problem 3E
Related questions
Question
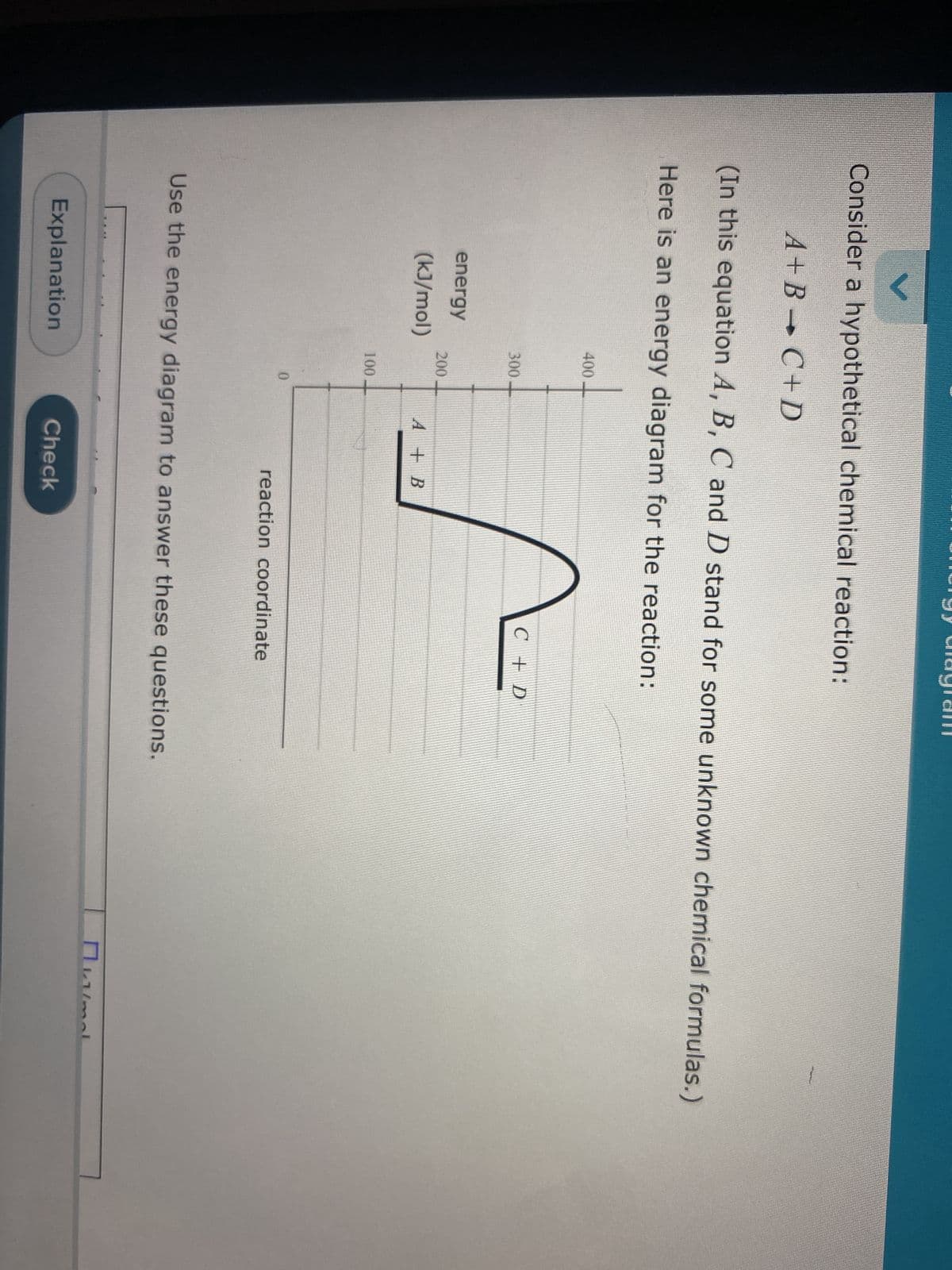
Transcribed Image Text:Consider a hypothetical chemical reaction:
A+B C+D
(In this equation A, B, C and D stand for some unknown chemical formulas.)
Here is an energy diagram for the reaction:
energy
(kJ/mol)
400
Explanation
300-
200
100
0
A + B
C + D
reaction coordinate
Use the energy diagram to answer these questions.
Check
Muumal
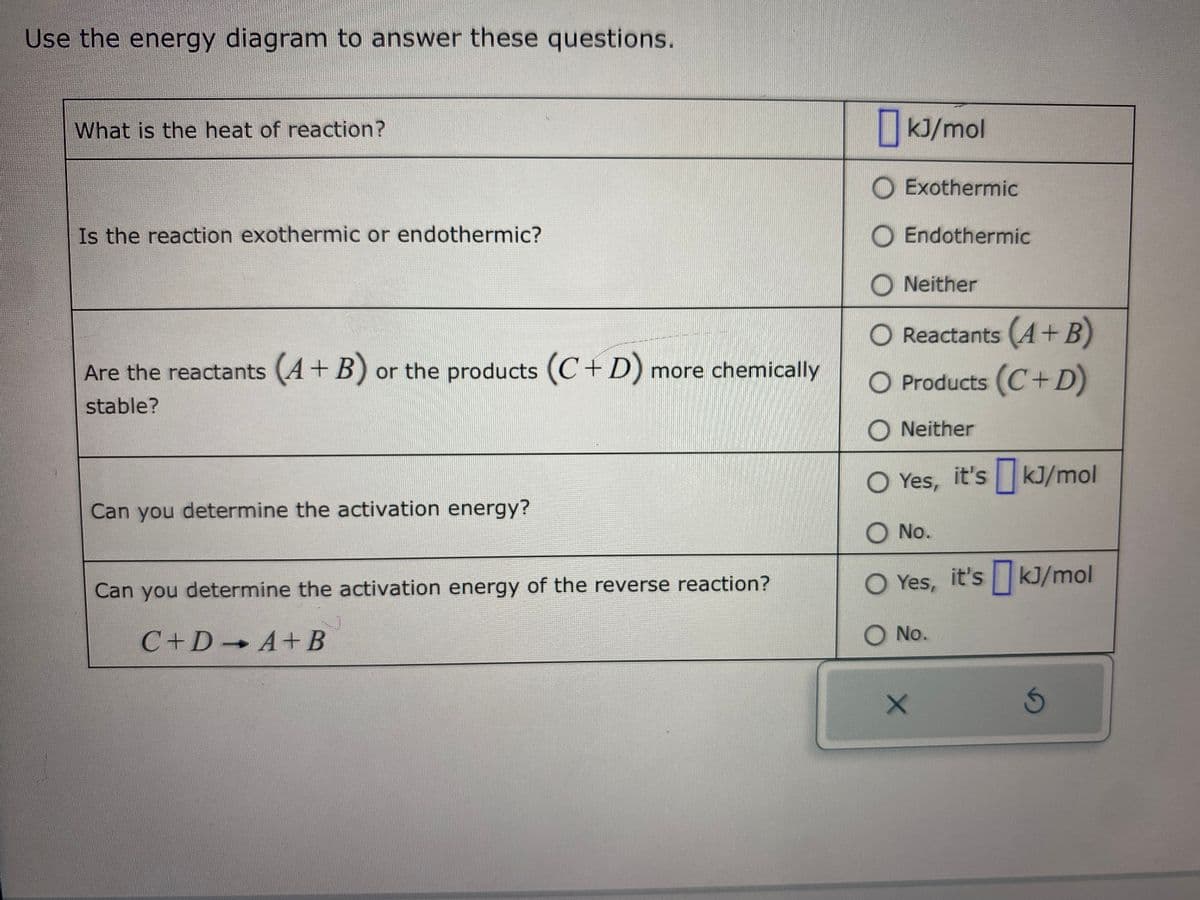
Transcribed Image Text:Use the energy diagram to answer these questions.
What is the heat of reaction?
Is the reaction exothermic or endothermic?
Are the reactants (A + B) or the products (C+D) more chemically
stable?
Can you determine the activation energy?
Can you determine the activation energy of the reverse reaction?
C+D+A+B
kJ/mol
Exothermic
O Endothermic
ONeither
O Reactants (A + B)
O Products (C+D)
ONeither
O Yes, it's kJ/mol
O No.
O Yes, it's kJ/mol
O No.
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning