Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 40QAP
Related questions
Question
Transcribed Image Text:ock
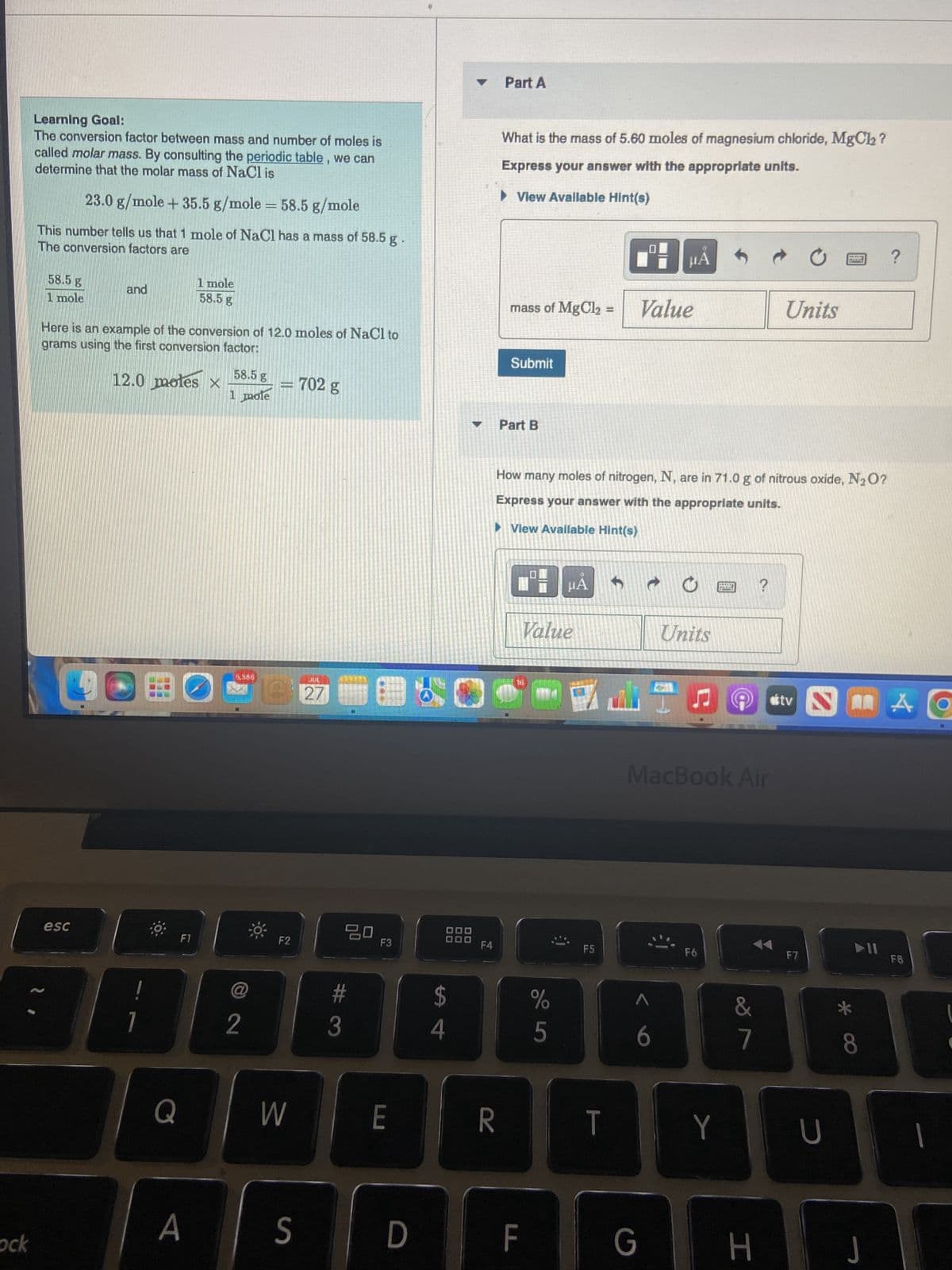
Learning Goal:
The conversion factor between mass and number of moles is
called molar mass. By consulting the periodic table, we can
determine that the molar mass of NaCl is
23.0 g/mole + 35.5 g/mole = 58.5 g/mole
This number tells us that 1 mole of NaCl has a mass of 58.5 g
The conversion factors are
58.5 g
1 mole
and
esc
Here is an example of the conversion of 12.0 moles of NaCl to
grams using the first conversion factor:
12.0 moles x
1
Q
1 mole
58.5 g
F1
A
58.5 g
1 mole
2
JE
-702 g
F2
W
S
JUL
27
20
#
3
F3
E
D
DO
$
4
F4
Part A
R
What is the mass of 5.60 moles of magnesium chloride, MgCl₂?
Express your answer with the appropriate units.
▶ View Avallable Hint(s)
mass of MgCl₂
Submit
Part B
F
How many moles of nitrogen, N, are in 71.0 g of nitrous oxide, N₂O?
Express your answer with the appropriate units.
►View Available Hint(s)
Value
%
са
HA
5
F5
T
μA
Value
6
G
Units
MacBook Air
F6
DELET
FORMARE
Y
&
7
?
H
Units
stv
F7
U
▶11
8
J
?
A
FB
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning