Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:S outo
Classes
e myBackpack
C Infinite Campus
9 New Tab
File| /media/fuse/drivefs-a644cf067c63cc0d1217e47dfd368497/root/k.g_k.sch_ZG9taW5pY28ua2FudG9yQGFwc2sxMi5vcmc_Whiteboar. Q < *
1 /1
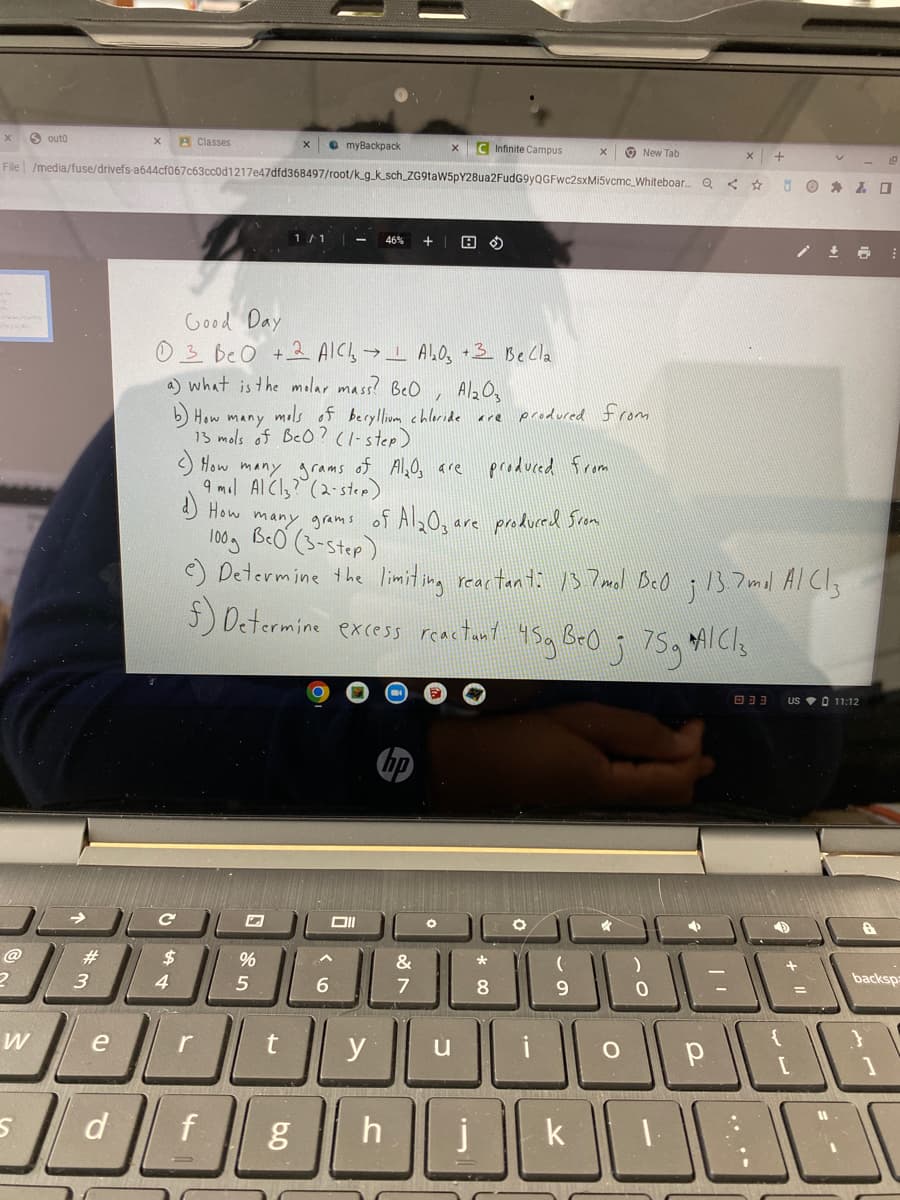
Good Day
03 Be O +2 AIC→I AbO, •3 Be Cla
a) what is the molar mass? BeO , AlzOa
b) How many mols of beryllium chleride are produced from
13 mols of BeO?(1-step)
) How many grams of Al,O, are
9 mol AICI,? (2-step)
) How many grams of Al2Og are produced Srom
100g BeO (3-step)
e) Determine the limit ing reartan ti 13 7mol BeO ;13.7mol Al Cl,
produced from
5) Determine excess reactant 4Sa BrO j 75q AICIS
US VO 11:12
@
%23
2$
%
4
7
8
backsp
W
e
y
d
h
k
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning