What is the ppm Zn in the orginal foor powder sample, after dissolving 50.0 ml of good water, the zinc in a 0.3232 gram sample of foot powder was titrated at a pH=11.0 with 19.67 ml of a 0.01222M EDTA solution
Q: A substance X was shown to be a weak acid with a single pKa value of 3.00. Using the substance, a…
A:
Q: What is the pH when 25% of the acid H3AsO4 (pKa = 2.22) has reacted with NaOH in a titration?
A: The reaction of H3AsO4 with NaOH results in the conversion of 25% of the acid into its conjugate…
Q: 1. A 0.0250 M Ca(X)₂2 has a pH of 8.049. Ca(X)2 is composed of a Ca²+ cation and an unknown anion X,…
A: Given: The concentration of the salt Ca(X)2 = 0.0250 M pH of the solution = 8.049 We have to find:…
Q: Bromothymol blue (MWt 624 g/mole, weak acid, pka 7.1) diluted solution is prepared by diluting 2.5…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: A 4.932-g sample of a petroleum product was burned in a tube furnace, and the SO2 produced was…
A: The reaction is given as: SO2(g)+H2O2→H2SO4 There is a reaction taking place between NaOH, H2SO4 and…
Q: Calculate using 0.0500M EDTA, a. volume needed to titrate the Mg in 0.1975 g of MgCO3 (84.305 g/mol)…
A: Given data,Molarity of EDTA=0.0500MMass of Mg=0.1975gMolar mass of Mg=84.305g/mol
Q: Calculate the volume, in milliliters, of a 0.780 M KOH solution that should be added to 4.500 g of…
A:
Q: A) Cyanide ion (CN') causes toxicity to humans, explain the reason of its toxicity to us. Suggest a…
A:
Q: after dissolving in 50.0 ml of good water, the zinc in a 0.3232 gram sample of foot powder was…
A: The percentage of Zn can be calculated asThe weight of zinc = 0.3232 gramsMolar mass of Zn2P2O7 =…
Q: 6mL of 125mM EDTA was added to 244mL of water. The resulting solution was added to 219mL of water…
A: MW of EDTA =292.24 g initial molarity of EDTA = 125mM
Q: Calculate the molar Y(4-) concentration in a 0.040M EDTA solution buffered to pH 11?
A: The ligand ethylenediamine tetra acetic acid form a strong complexe with many metal ion. It is an…
Q: 19. A 0.01086 M EDTA solution is used to titrate a 100 mL sample of water, adjusted to pH 10, using…
A:
Q: An EDTA solution was prepared by dissolving approximately 4 g of the disodium salt in approximately…
A: Given mass of MgCO3 = 0.07682 g Volume of water = 1 L Molar mass of MgCO3 = 84.31 g/mol
Q: As part of a geological team that studied a local cave, you brought with you a bunch of 1.00 g rock…
A: Cacite is also called as Calcium carbonate (CaCO3). Its abundance is very high and is referred as…
Q: A 0.25-mol sample of a weak acid with an unknown pKa is combined with 10.0 mL of 3.00 M KOH and…
A: Given,A 0.25-mol sample of a weak acid with an unknown pKa is combined with 10.0 mL of 3.00 M KOH…
Q: If a 50.00 mL sample of 0.127 M nitrous acid is titrated with 17.55 mL of 0.102 M NaOH, what is the…
A:
Q: A solution of nitrous acid (0.162 M, 23.82 mL) was titrated with 0.124 M sodium hydroxide (Ka = 4.00…
A:
Q: 0.28g Of MBr is dissolved in distilled water and made up to 250mL mark in a volumetric flask. 25 mL…
A: The equation to calculate the unknown values from known values is given as, M1V1=M2V2 where M is the…
Q: A solution was prepared by dissolving about 30.00m g of EDTA in approximately 1 L of water and…
A: The solution is given below -
Q: For the following, calculate the hydroxide (OH"), carbonate (CO,²) and bicarbonate (HCO3) alkalinity…
A:
Q: A 0.72g sample of foot powder containing Zinc was titrated with 21.3mL of 0.02M EDTA. Calculate the…
A:
Q: A sample of water (200 mi.) was titrated by 0.05 M HZS04, The bitration completed when the color…
A:
Q: A 1.7483-g sample containing Al(NO3)3, AlCl3, and inert material was dissolved in acid and divided…
A:
Q: An antacid tablet, weighing 1.25 g, was dissolved in a 1.0 L volumetric flask to allow for…
A: Given: Mass of tablet=1.25g Volume of solution=1.0L Volume of aliquot 10.0mL Molarity of…
Q: A 0.2431 g sample of CaCO3 is dissolved in 6 M HCl and the resulting solution is diluted to 250.0…
A: Mass of sample=0.2431 g Molar mass of CaCO3= 100.08 g /mol Titrated volume = 25 mL Total volume =…
Q: An EDTA solution was prepared by dissolving approximately 3 grams of Na2H2Y•H2O in sufficient water…
A: Given: Volume of Mg2+ ions = 50.00 mL = 0.050 L Molar concentration of Mg2+ = 0.004517 M Volume of…
Q: Calculate the solubility, s, of Mg(OH)2 (s) in grams per liter in an aqueous solution buffered at…
A:
Q: As part of a geological team that studied a local cave, you brought with you a bunch of 1.00 g rock…
A: The reaction taking place will be, MgOH2+EDTA4- → MgOH2EDTA4- Calculate number of moles of EDTA: 1…
Q: An antacid tablet, weighing 1.25 g, was dissolved in a 1.0 L volumetric flask to allow for…
A: Given data,Mass of tablet=1.25gVolume of solution=1.0LVolume of aliquot=10.0mLMolarity of…
Q: 0.28g 0f MBr is dissolved in distilled water and made up to 250mL mark in a volumetric flask. 25 mL…
A: The titration of MBr by AgNO3 is a double-displacement reaction, which is given by,…
Q: Calculate the volume of 0.0602 M EDTA needed to titrate 11.63 mL of 0.0725 M Mg(NO3)2.
A:
Q: If 10.00 mL sample of 0.01 M standard Ca2+ solution is titrated with 9.8 mL of EDTA solution, what…
A: In a Calcium-EDTA titration both reacts in equal proportion. EDTA itself is not so soluble so its…
Q: 10 Calculate the pH at the equivalence point when 0.104 g sodium acetate (MM-82.0,Kb-5.6x10-¹0) Is…
A: #10.(a): The balanced equation for the reaction of sodium acetate, NaCH3COO(aq) and HCl(aq) is:…
Q: The Zn in a 0.6803-g sample of foot powder was titrated with 27.3 mL of 0.04246 M EDTA. Calculate…
A: To calculate the percentage of zinc in the foot powder sample, use the EDTA titration. First…
Q: 4.912-g sample of a petroleum product was burnedin a tube furnace, and the SO2produced was…
A:
Q: if a 1.25g sample of magnesium oxide (98.9%) were titrated with 70ml of 1.04N of sulfuric acid, what…
A:
Q: What is the pH of the solution that results from the mixing of 250.0 mL of 0.350M HCl and 100.0 mL…
A:
Q: Given 0.10 M solutions of acetic acid (pKa = 4.76) and sodium acetate, describe how you would go…
A: The volume of acetic acid and acetate to be mixed is calculated as, pH = pKa + log ([A-]/[HA]) [A-]…
Q: Calculate the pH of: A 0.0850 molar solution of the intermediate (HA) form of threonine. It is…
A: Given, 0.0850 molar threonine compounds The pOH of the solution is calculated as follows,
Q: n alkaline sample of sodium compounds weighing 1.196 g was dissolved in water, cooled to 15°C,…
A: Given: Mass of alkaline sample of sodium compounds= 1.196 g Normality of H2SO4= 1.058 N The volume…
Q: 0.8153 g of a sample containing Pb(NO3)2 was taken, dissolved in water, and 40.20 mL of 0.06 M EDTA…
A: A numerical problem based on EDTA titration, which is to be accomplished.
Q: A 14.92 g sample of bacon is pureed in a blender with 100.0 mL of water. The suspension is filtered…
A: First we balance the redox reactions involved in the above process
Q: Calculate the sulfur concentration in parts per million.
A:
Q: If a sample of silver coin weighing 0.5230 g gives a precipitate of AgCl (143.45) weighing 0.3559 g,…
A: The mass of the silver coin sample is = 0.5230 g The mass of the AgCl precipitate is = 0.3559 g The…
Q: which of the following is the concentration of the EDTA solution in terms of molarity
A: Solution: Here given experimente is carried out to know concentration of EDTA solution. Where,…
Q: A 4.908-g sample of a petroleum product was burned in a tube furnace, and the SO2 produced was…
A:
Q: A) Cyanide ion (CN') causes toxicity to humans, explain the reason of its toxicity to us. Suggest a…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Given that the titration of Ca2+ and Mg2+ in a 50.00-mL sample of hard water required 22.35 mL of…
A:
Q: 0.316 grams of a sample containing chloride is taken and treated with an excess of 48.5 mL of 0.11 M…
A: Here we are required to find the percentage of chloride present in the sample
Q: 0.8153 g of a sample containing Pb(NO3)2 was taken, dissolved in water, and 40.20 mL of 0.06 M EDTA…
A: The question is based on the concept of complexometric titrations. we have to calculate mass…
What is the ppm Zn in the orginal foor powder sample, after dissolving 50.0 ml of good water, the zinc in a 0.3232 gram sample of foot powder was titrated at a pH=11.0 with 19.67 ml of a 0.01222M EDTA solution
From Gas law
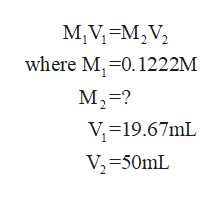
Now calculate the molarity of Zn solution
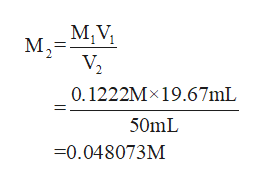
Step by step
Solved in 4 steps with 3 images

- after dissolving in 50.0 ml of good water, the zinc in a 0.3232 gram sample of foot powder was titrated at a pH=11.0 with 19.67 ml of a 0.01222M EDTA solution. calculate the %Zn in this sampleCalculate the volume, in milliliters, of a 0.770 M KOH solution that should be added to 5.000 g of HEPES (MW = 238.306 g/mol, pKa = 7.56) to give a pH of 7.50. KOH volume = mLAn antacid tablet, weighing 1.25 g, was dissolved in a 1.0 L volumetric flask to allow for determination of calcium carbonate and magnesium carbonate contents. After preparing the solution, an aliquot of 10.0 ml was transferred to an Erlenmeyer flask containing a buffer solution with pH = 10. This aliquot was then titrated with EDTA 0.0040 mol/L and the average volume spent was 15.01 mL. To measure calcium after precipitation After the magnesium was fractionated, a second 10.00 mL aliquot of the stock solution was transferred to an Erlenmeyer flask. and the pH of the present solution was adjusted to a value of approximately 13. In EDTA titration, the volume consumed for observation of the end point was equal to 13.03 mL. Based on this information, (a) Outline the two steps involved, representing the related reactions. (b) Calculate the concentrations of CaCO3 and MgCO3 present in the initial solution. (c) Calculate the masses of CaCO3 and MgCO3 present in the pellet. (d) Calculate the…
- . An antacid tablet, weighing 1.25 g, was dissolved in a 1.0 L volumetric flask to allow for determination of calcium carbonate and magnesium carbonate contents. After preparing the solution, an aliquot of 10.0 ml was transferred to an Erlenmeyer flask containing a buffer solution with pH = 10. This aliquot was then titrated with EDTA 0.0040 mol/L and the average volume spent was 15.01 mL. To measure calcium after precipitation. After the magnesium was fractionated, a second 10.00 mL aliquot of the stock solution was transferred to an Erlenmeyer flask, and the pH of the present solution was adjusted to a value of approximately 13. In EDTA titration, the volume consumed for observation of the end point was equal to 13.03 mL. Based on this information, a) Outline the two steps involved, representing the related reactions./5 b) Calculate the concentrations of CaCO3 and MgCO3 present in the initial solution./6 c) Calculate the masses of CaCO3 and MgCO3 present in the pellet./6 d) Calculate…Calamine, which is used for relief of skin irritations, is a mixture of zinc and iron oxides. A 1.022-g sample of dried calamine was dissolved in acid and diluted to 250.0 mL. A 50.00 mL aliquot was suitably buffered and titrated with 2.40 mL of 0.002727 M ZnY-2 (Zn-EDTA) solution to allow the following reaction: Fe+3 + ZnY-2 → FeY- + Zn+2. Molecular mass: Fe2O3 = 165.74 a. The weight of sample in the aliquot portion is _________g ? b. the percentage composition of Fe2O3 in the sample is ______ % ?0.28g 0f MBr is dissolved in distilled water and made up to 250mL mark in a volumetric flask. 25 mL of this solution is titrated against 0.019 M silver nitrate solution using eosin as indicator. The end point was obtained at 12.4 mL of AgNO3 solution . Calculate the molar mass of the metal bromide.
- For complexometric titration of Ca (II) ions in the shell of an egg sample weighing 59,427 g, the necessary processes were applied to the egg shells and a sample of 100 mL was prepared. From the prepared sample, 1 mL was taken and diluted to 100 mL, the pH was adjusted, and two drops of EBT indicator were dripped on it. The prepared solution was titrated with 27.4 mL of EDTA solution set at 0.0097 M. Calculate the amount of calcium in the sample in CaCO3%. (Ca = 40g / mol, CaCO3= 100g / mol)50 mL of a sample known to contain Mg+2 was taken and titrated with 0.075 M EDTA standard solution by adding pH 10 buffer and EBT indicator. Since the EDTA consumption is 10.5 mL, what is the amount of magnesium in the sample in ppm?Titration of 25.0 mL of a 0.0500 M Zn2+ solution with 0.0550 M EDTA in a solution buffered at pH 8. Assume that the temperature is 25 oC and that the formation constant for Zn2+ is 3.13 x 1016 at this temperature. What is the pZn of the solution after 30 mL of titrant have been added?
- Titration of 25.0 mL of a 0.0500 M Zn2+ solution with 0.0550 M EDTA in a solution buffered at pH 8. Assume that the temperature is 25 oC and that the formation constant for Zn2+ is 3.13 x 1016 at this temperature. What is the pZn at the equivalence point of the titration?An EDTA solution was prepared by dissolving approximately 3 grams of Na2H2Y•H2O in sufficient water to give 1 liter of solution. This solution was then standardized against 50.00 mL aliquots of 0.004517 molar Mg2+. An average titration volume of 32.22 mL was required. Determine the molar concentration of EDTA.If a 50.00 mL sample of 0.127 M nitrous acid is titrated with 17.55 mL of 0.102 M NaOH, what is the pH of the titration mixture? (For HNO2, Ka = 5.62 x 10-4)



