Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.19QAP
Related questions
Question
100%
is the volume needed to titrate the Vequivalence?
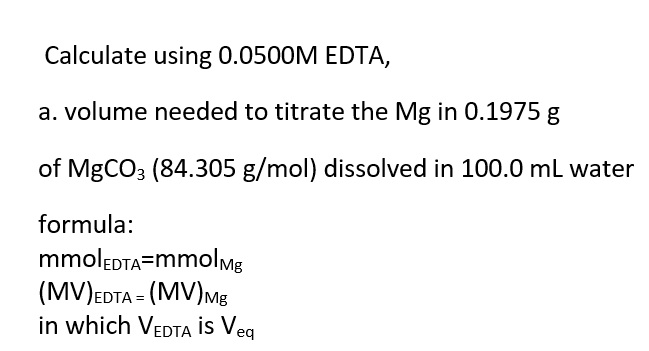
Transcribed Image Text:Calculate using 0.0500M EDTA,
a. volume needed to titrate the Mg in 0.1975 g
of MgCO3 (84.305 g/mol) dissolved in 100.0 mL water
formula:
mmolEDTA=mmolmg
(MV)EDTA = (MV)Mg
in which VEDTA İs Veq
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole