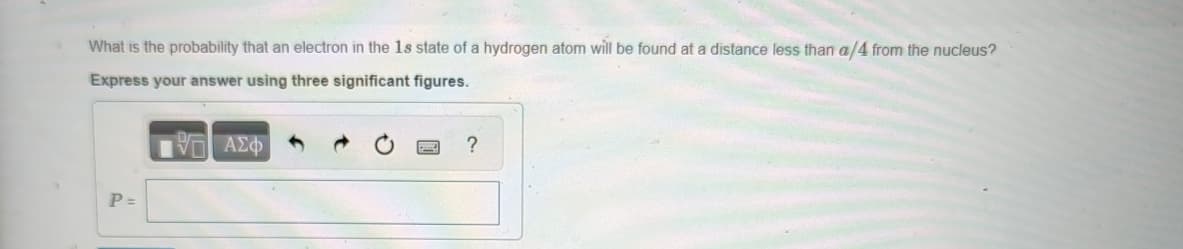
What is the probability that an electron in the 1s state of a hydrogen atom will be found at a distance less than a/4 from the nucleus? Express your answer using three significant figures. ΜΕ ΑΣΦ P = ?
Q: I would like to have a set of ideas for improving ventilation inside gyms or improving sound…
A: Improving ventilation in gyms involves implementing advanced HVAC systems with HEPA filters to…
Q: 2) Determine the dew point of moist air at 80 F (27 C) and 60 percent relative humidity for…
A: Step 1: Step 2:
Q: Pravinbhai
A: The objective of the question is to determine the acceleration of point D in a slider-crank…
Q: Please show all your work and explain in details. I am prepping for my exam tomorrow and I am stuck…
A: The objective of this problem is to determine the minimum shaft diameter for ABCD based on strength…
Q: Divide the plane frame structure shown in the image into suitable parts for analytical examination…
A: Step 1:Step 2:Step 3:
Q: Please help. I am not sure how to approach this problem. This problem involves heat transfer and…
A: The objective of the question is to determine the mean air temperature as a function of distance…
Q: PROBLEM 3: The tank shown in the figure weighs 3.8 kN. In this problem, D = 1.5 m, H = 3.4 m and e…
A:
Q: Can anybody help me, the underlying reason behind the equations of teta 1 up until 6. These…
A: The objective of the question is to understand the underlying reason behind the equations of theta 1…
Q: Consider a system whose model is given by m dx²(t) dt² +c dx(t) dt -+kx(t)=5, where m = 1, c = 5,…
A:
Q: In orthogonal cutting, __________ are independent variables. I. Surface finish of cutting tool;II.…
A: The objective of the question is to identify the independent variables in orthogonal cutting.…
Q: solve for given solutions
A: Step 1:Step 2:Step 3:Step 4:
Q: The truss in the figure below is under the effect of one load, this truss consists of three parts,…
A: “Since you have posted a question with multiple sub parts, we will provide the solution only to the…
Q: y x = r cos 0 V = Or y = r sine r = √x² + y² χ Flow in "solid body rotation" acts like a solid…
A: Solution: The curl of the given velocity vector is zero.
Q: The aluminum block has a rectangular cross section and is subjected to an axial compressive force of…
A: if you have any doubt comment
Q: Find the reactions at the fixed support A given the distributed loads shown. (+)+ 1 = (d) 1 = su A y…
A: Step 1:Step 2: Step 3: Step 4:
Q: An orifice in a vertical plate is located 1.13m below surface of an oil. The area of the orifice is…
A:
Q: Arm AB has a constant angular velocity of 28 rad/s counterclockwise. At the instant when 0 = 60°,…
A: Given that:Angular Velocity : 28 rad/secθ=90o We will solve for the acceleration of collar…
Q: Pravinbhai
A:
Q: Please answer the attached question which includes all the necessary background information sourced…
A: The objective of the question is to find the value and location of the largest tensile and…
Q: Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it…
A:
Q: how does your computations go when for example all needed info are derived as usual and THE PIPE…
A: Introduction-Fluid movement occurs often during solidification, interfering with the previously…
Q: A two-dimensional flow field has velocity components u = x v = by (a) For which value of b is the…
A: Step 1:Step 2: Step 3: Step 4:
Q: Parvinbhai
A: The objective of the question is to solve the given second order differential equation with the…
Q: A turbine rotor of a ship is of 2000 kg mass and has a radius of gyration of 0.8 m. Its speed is 200…
A: The objective of the question is to determine the maximum couple tending to shear the holding down…
Q: solve show solutions
A: Step 1:Diameter is 6cmCoefficient of friction is 0.2The mass of the bucket is 100kgAngle of the pipe…
Q: 2-29 For the bracket shown in Figure P2-14 and the data in the row(a) assigned from Table P2-3,…
A: The objective of this question is to calculate the von Mises stresses at points A and B for the…
Q: Locate the center of gravity of the two-block assembly. The specific weights of the materials A and…
A: Step 1:Step 2:Step 3: Step 4:
Q: Determine the normal forces in the bars BC,BG,FG, use the method of sections.
A:
Q: 3- A large plane wall has a thickness L=50 cm and thermal conductivity k=25 W/m.K. On the left…
A:
Q: Crack Strain Energy Release Rate in Cracked Tension Rod. In the below, the crack growth in an…
A:
Q: Pravinbhai
A: rC/A={0.5i+0.5j}vC=−vCivC=vA+Ω×rC/A+(vC/A)xyz−vCi=0+(5k)×{0.5i+0.5j}+(vC/Acos45i+vC/Asin4…
Q: 3.3. The velocities at a point in a fluid in the Eulerian system are given by ux+y+z+s =2(x + y + z)…
A: Please Rate if this solution helps youThank You
Q: A mass of 1.5 kg is suspended from a swivel point by a string of 1.2m long. The mass revolves in a…
A: Based on the information provided and the image you sent, we can analyze the situation to determine…
Q: Learning Goal: To determine the in-plane principal stresses and maximum shear stress along with…
A: Step 1: Step 2: Step 3: Step 4:
Q: A ball of mass m is sliding in a frictionless tube of total length 4L as shown below in the figure.…
A:
Q: Three types of grains are used to make a grain mixture: wet oats (20% moisture by mass), wet corn…
A:
Q: 5. A particle moves with simple harmonic motion between two points 1 m apart. The frequency of the…
A: Approach to solving the question: Simple harmonic motion. Detailed explanation:
Q: 3. From the results you obtained in this laboratory, compute the net muscle force in the biceps…
A: Approach to solving the question:To compute the net muscle force in the biceps brachii necessary to…
Q: Water squirts from a 10 mm diameter domestic tap. The flow is found to fill a 1 litre jug in1.5…
A: The objective of the question is to calculate the power available from the flow of water from a tap.
Q: Hi, can you please help me with this problem?
A:
Q: 0:45] am- Google Chrome du/mod/quiz/attempt.php?attempt=2340155&cmid=742781 P 250 mm 400 mm Time…
A: **Approach to solving the question:**1. **Calculate the moment of inertia (Ia):** For a spool with a…
Q: Water travels through a circular pipe with a diameter of 400 mm and at a speed of 5 m/s. The…
A: Step 1: To calculate the inertial force created by the flow of water through the pipe, we can use…
Q: Draw the velocity triangles at the inlet and outlet sections of a centrifugal pump whose impeller…
A: The velocity triangles at the inlet and outlet sections of a centrifugal pump whose impeller…
Q: STOP USING STUPID ARTIFICIAL INTELLIGENCE
A: Given information:Constant bending stiffness (EI) for the beamLength of beam sections: AB = 15 ft,…
Q: Please answer all parts
A: Step 1: Step 2:Step 3: Step 4:
Q: Workplace violence is relatively insignificant compared to other risks and has little impact on the…
A: The objective of the question is to understand the significance of workplace violence and its impact…
Q: What is the difference between thermoplastics and thermosets? Select one: O a. There is no…
A: Thank you.
Q: Pravinbhai
A: Step 1: Step 2: Step 3:
Q: A concrete-lined channel is used to convey water flow between Point A at surface elevation of 510 m…
A: Step 1:Step 2:
Q: 60 mm 20 mm 10 mm 20 mm 60 mm 10 mm 100 mm- 10 mm- PROBLEM 9.71 Using the parallel-axis theorem,…
A: the circuit has an open switch (S1). In an open circuit, current flow is interrupted by the open…
Step by step
Solved in 2 steps with 2 images

- In the ground state of cadmium, Cd,a. how many electrons have l = 2 as one of their quantum numbers?b. how many electrons have n = 4 as one of their quantum numbers?c. how many electrons have ml = −1 as one of their quantum numbers?d. how many electrons have ms =-1/2 [as one of their quantum numbers?Polarizability is defined as the extent to which the electron cloud surrounding an atom or molecule can be distorted by an external charge. Rank the halogens (F2,Cl2,Br2,I2) and the noble gases (He,Ne, Ar, Kr, Xe) in order of polarizability (from least polarizable to most polarizable). What characteristics of these substances could be used to determine this ranked order?14- Which of the following is constant during Atomic Packing Factor (APF) calculation a. All of the above b. Volume of an atom c. Number of effective atoms per unit cell d. Volume of unit cell
- Which of the following have the same periodicity as the crystal lattice: a) electron wave function, b) electron distribution probability, c) lattice potentialQ: Not considering the many subatomic particles, name the three basic components of which atoms are made. What differences are there among the three? A: The three (3) basic components of which atoms are made, The ___________, which has a positive (+) electrical charge, and the _____________ , which has a neutral electrical charge, make up the nucleus. ___________ are particles of negative (-) electric charge which surround the nucleus in shells or orbitals.Why do quantum effects only happen on the atomic scale?
- Consider a system of nonrelativistic electrons in a white dwarf star at a temperature of 10^9 K. Very roughly, what would be their density if the system is degenerate? How does this compare with typical electron densities in ordinary matter of about 1030 electrons/m3?If the energy for Frenkel defect formation in Silver Chloride is 1.1 eV, the number of Frenkel defects at 350°C will be (density of AgCl is 5.50 g/cm^3, atomic masses of Ag & Cl are 107.87 g/mol & 35.45 g/mol, respectively) K=8.62 × 10^-5 eV/K) 6.56 × 10^23 /m^3 6.56× 10^23/m^3 8.24 × 10^23 /m^3 8.24× 10^23 /cm^34. Consider a binary alloy of A atoms and B atoms that can exist in a solid phase or a liquid phase. This alloy can also exist in TWO DIFFERENT PHASES SIMULTANEOUSLY: solid and liquid. The system is composed of one mole of atoms, some of which are A atoms and some of which are B atoms. Take GA and GB as the molar Gibbs free energy of pure A and pure B respectively. XA and XB are the molar fractions of A and B atoms respectively. The atoms crystallize in identical crystal structures when they are solid. (a) The starting conditions are such that a partition sits between the A atoms and B atoms so they do NOT mix. (i) Write an expression for the molar Gibbs free energy for the combination of pure components. (ii) Draw schematically how the molar Gibbs free energy of the combination of pure components varies with alloy composition.

