Q: After mixing these solutions... determine which compound, (NazCO3 or Ni(NO3)2) will be the limiting…
A: Calculation of number of moles of the two reactants given is shown below.
Q: A 1.00 gg sample of potassium bicarbonate is decomposed by heating. If the resulting potassium…
A: Given, A 1.00 g sample of potassium bicarbonate is decomposed by heating. Actual yield = 0.710g…
Q: A LEVEL teaspoon of baking soda is equivalent to 4.80 grams. Utilizing the power of stoichiometry,…
A:
Q: Explain why the statement, “The limiting reactant is thereactant with the lowest mass” is incorrect.
A: Limiting reactant is the reactant which completely consume in the reaction after some time and then…
Q: What does it mean to say a reactant is present “in excess” in a process? Can the limiting reactant…
A: Limiting reactant: In a chemical reaction the substance which is totally consumed is known as…
Q: The percent yield for the reaction PCI3 + Cl2 → PCI5 is 58.1 percent. What mass of PCI5 would be…
A:
Q: When copper is heated with an excess of sulfur, copper(I) sulfide is formed. In a given experiment,…
A:
Q: Calculate the percentage by mass of the indicated element in the following compounds: oxygen in…
A: Molar mass of SO2 is 64.06 g/mol Atomic mass of Oxygen is 16.00 g/mol
Q: In this reaction: Mg (s) + I, (s) → Mgl, (s) If 2.34 moles of Mg react with 3.56 moles of I,, and…
A: Given, Mg +I2 ----> MgI2 No. of moles of Mg = 2.34 mol No. of moles of I2 = 3.56 mol To…
Q: When 3.16 g of N2 is allowed to react with an excess of Li2O, 1.28 g of Li3N are produced. What is…
A:
Q: If 1.7 moles of ammonium phosphate reacts with zinc nitrate, what will be the actual yield (in…
A: 2(NH4)3PO4 + 3Zn(NO3)2 → Zn3(PO4)2 + 6NH4NO3 From this Balanced chemical equation we get that ;…
Q: (a) Define the terms limiting reactant and excess reactant.(b) Why are the amounts of products…
A:
Q: Your theoretical yield is 63.247 g. You isolated 13.5648 g of alum. What is your percent yield?
A:
Q: what is the percent yield of this reaction?
A: Given :- C(s) + 2 Cl2(g) ----> CCl4(l) Theoretical yield of CCl4 = 31.363 g Actual yield of…
Q: A 1.50 gg sample of sodium nitrate is decomposed by heating. If the resulting sodium nitrite has…
A: Given: The actual yield of NaNO2, = 1.22 g. The theoretical yield of NaNO2, = 1.32 g.
Q: The percent yield for a given reaction is 47%. If the theoretical amount is predicted to be 5.29 g,…
A: Formula for calculating percent yield: Percent yield = 100 x Actual yield/Theoretical yield
Q: The combustion of 0.374 kg of methane in the presence of excess oxygen produces 0.983 kg of carbon…
A:
Q: when copper is heated with an excess of sulfur, copper (i) sulfide is formed. in a given experiment,…
A: Here we have to calculate percentage of yield of copper(1) sulfide-
Q: What experimental information do you need in order tocalculate both the theoretical and the percent…
A: To calculate the theoretical yield of any chemical reaction, we need to know the quantity of one of…
Q: A reaction has a theoretical yield of 76.5 grams of a product, and a percent yield of 32.5 %. What…
A: A numerical problem based on percent yield, which is to be accomplished.
Q: An intial mass of 1.025g sodium carbonate was used. What is the theoretical yield in the reaction…
A:
Q: When copper is heated with an excess of sulfur, copper(I) sulfide is formed. In a given experiment,…
A: Percent yield is defined as the percentage ratio of the actual yield to the theoretical yield…
Q: - Although they were formerly called the inert gases, at least the heavier elements of Group 8 do…
A:
Q: d. How many grams of phosphorus pentachloride are theoretically produced? If 0.450g of phosphorus…
A: From the given problem, the balanced equation is: P4 (s) + 10 Cl2 (g) → 4PCl5 (s) For chlorine gas…
Q: The theoretical yield of a reaction is 75.0 grams of product and the actual yield is 42.0 g. what is…
A: Given as The theoretical yield of a reaction is 75.0 gram and actual yield is 42.0 g % yield has to…
Q: Titanium is a metal with the same streangth as steel but 45% lighter.It is also resistant corrosion…
A:
Q: A 0.4505 g sample of aluminum reacts according to our experiment to produce alum. 5.7955 g of dried…
A: Given : Mass of Al reacting = 0.4505 g And mass of Alum produced = 5.7955 g Molecular formula of…
Q: What is the maximum amount of sodium chloride that can be formed? grams What is the FORMULA for the…
A: Those reagent which is less are limiting reagent.
Q: grams
A:
Q: a student completely reacts 12.5g of calcium w an excess of oxygen to produce calcium oxide .…
A: The number of moles of a species is calculated by the expression (1) in which n is the number of…
Q: If the actual mass of a product formed in a reaction is 12.75 g less than the theoretically…
A: Given, Theoretical mass = 25.69 g Actual mass is 12.75 g less than the theoretical mass.
Q: When copper is heated with an excess of sulfur, copper(I) sulfide is formed. In a given experiment,…
A: Given: Moles of Copper = 0.0970 moles Mass of Copper sulfide = 1.55 g Molar mass of copper sulfide =…
Q: What is the percent yield if 0.5177 g of Al reacts completely and 7.7101 g of alum is recovered?
A:
Q: Use the References to access important values if needed for this question. According to the…
A:
Q: When copper is heated with an excess of sulfur, copper(I) sulfide is formed. In a given experiment,…
A:
Q: When 6 moles of H₂ reacted with 3 moles of N₂ to produce, NH₃, which is the limiting reactant? *
A: Answer: In chemical reaction, limiting reagent is the reactant that exhausted first and decided the…
Q: In a reaction to produce sulfuric acid, the theoretical yield is 300. g. What is the percent yield…
A: Therotical yield of H2SO4 = 300 g Actual yield of H2SO4= 111.4 g
Q: Choose the key item that must be determined experimentally in order to calculate the percent yield…
A: The percent yield is calculated using the following formula:
Q: Consider the following unbalanced equation for the combustion of pentane: if a 20.4 g sample of…
A: Given that 20.4 g pentane react with excess oxygen.
Q: For the following reaction, 6.52 grams of sulfuric acid are mixed with excess barium hydroxide. The…
A: Given :- amount of sulfuric acid = 6.52 g actual yield of barium sulfate = 11.7 g To be…
Q: c) How many grams of the excess reactant remain after the limiting reactant is completely consumed?…
A: limiting reagent is completely consumed in a reaction, the maximum amount of yield that is the…
Q: According to the following reaction, how many moles of bromine monochloride will be formed upon the…
A: Given: Mass of Br2 = 32.8 g Molar mass of Br2 = 159.808 g/mol
Q: In a reaction to produce ammonia, the theoretical yield is 420.0 g. What is the percent yield if the…
A: Percent yield of the reaction is equal to the ratio of actual yield and theoretical yield multiplied…
Q: 5) 15.3 grams of Lithium is dropped into a solution containing excess copper II phosphate. When the…
A: 5. Given :- Mass of Li = 15.3 g Actual yield of Cu = 1.25 g To calculate : Percent…
Q: An excess of sodium carbonate, Na, CO,, in solution is added to a solution containing 19.25 g CaCl,.…
A: Percentage yield is a measure of the quantity of moles of a product formed in relation to the…
Q: What is the Actual yield and Percentage yield of calcium hydroxide?
A: Let᾿s take a chemical reaction in which X & Y are combined to generate Calcium hydroxide…
Q: The percent yield for a difficult reaction to be studied was likely to be only 51%of the theoretical…
A:
Q: Balance and complete the following combustion reaction. Enter the sum of the balanced coefficients…
A: Chemical reactions are those reactions which undergo any chemical change. Chemical reaction is…
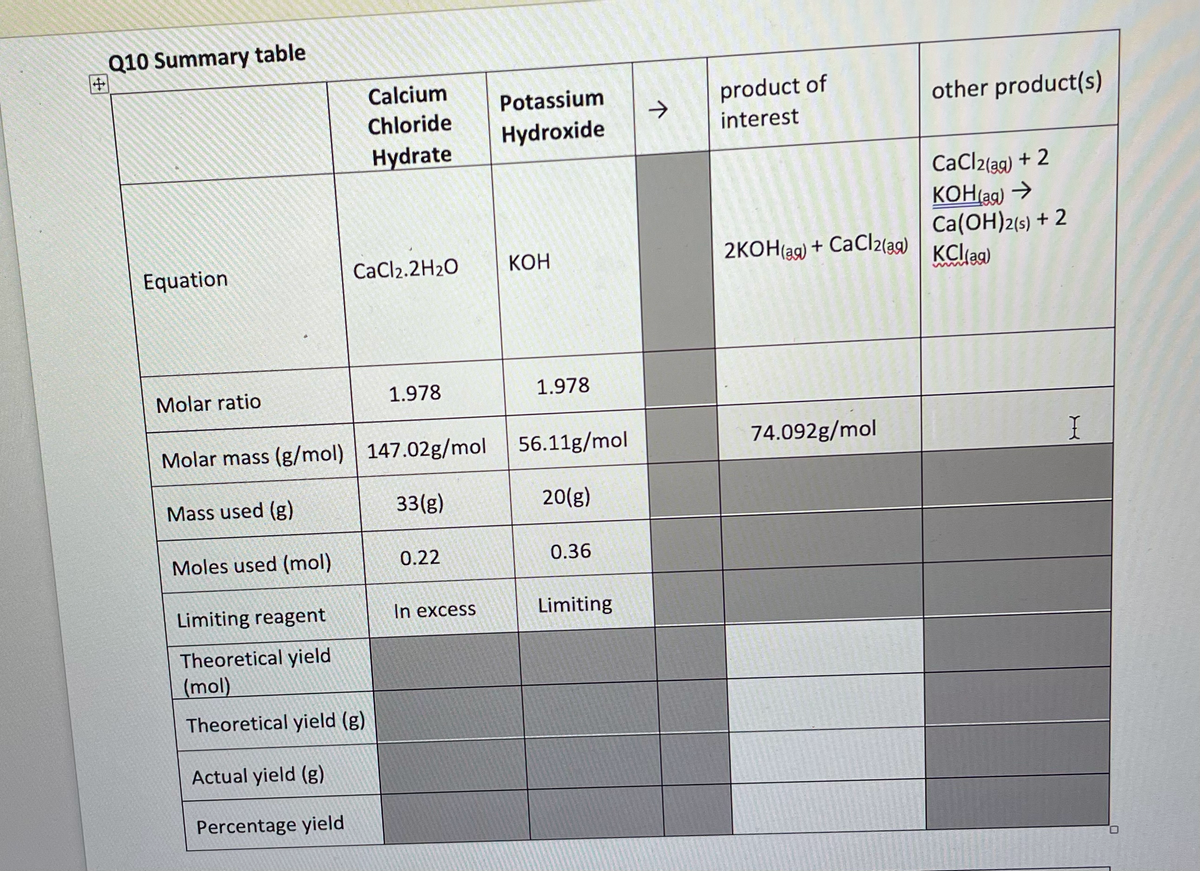
What is the 'Theoritical Yield' in moles and grams of calcium hydroxide
Step by step
Solved in 2 steps

- Which processes shown in this figure involve the phasetransition H2O(l )----->H2O(g)?Calculate the solubility of lead(II) sulfate (Ksp = 2.53x10-8) in a 0.0034 M solution of sodium sulfate. Give your answer to three sig. figs. and in exponential form (e. g. 1.23E-3).In the synthesis of hydrocarbons, the carbon source is carbon dioxide. Although the CO2 concentra?on in the atmosphere raises at a drama?c speed, point sources are probably the easier sources for a PtX process. Iden?fy 3 possible point sources, explain why CO2 is formed and what challenges each of the three CO2 streams presents
- The vapor-liquid reactive equilibria for ethyl lactate synthesis follows the expressionlog K = 7.893 – (2.4312 x 103)K/Tbetween 83.12 °C and 101.54 °C. What are the ΔH°rxn, ΔG°rxn, and ΔS°rxn at 85 °C?Ind. Eng. Chem. Res. 2008, 47, 5, 1453–1463Reducing NO Emissions Adding NH3 to the stack gases at an electric power generating plant can reduceNOx emissions. This selective noncatalytic reduction (SNR) process depends on the reaction between NH3 (an odd-electron compound) and NO.$$4NH3(g)+6NO(g)5N2(g)+6H2O(g)The following kinetic data were collected at 1200 K. Experiment [NH3] (M) [NO] (M) Rate (M/s) 1 1.00x10-5 1.00x10-5 0.120 2 2.00x10-5 1.00x10-5 0.240 3 2.00x10-5 1.50x10-5 0.360 4 2.50x10-5 1.50x10-5 0.450 What is the rate-law expression for the reaction? Do not add multiplication symbols to your answer. $$Rate=Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07
- 1. The Ksp of Ca3(PO4)2 is 1.3 × 10−26. Estimate the solubility of this salt in units of g. L−12. If a sample of solid Ca3(PO4)2 is stirred into exactly one litre of a 0.550M solution of Na3PO4, how will the solubility of the salt compare with the answer that you have obtained in question 2.1? Explain you answer in a short sentence. Please only answer 2nd QuestionThe equilibrium constant for the reaction below is 0.02 mol dm-3 at 298.000 K.Fructose 1,6-diphosphate _ glyceraldehyde 3-phosphate + dihydroxyacetone phosphateCalculate ΔG°.Provide answer to two decimal places and as J mol-1.If all other variables were kept constant, determine theeffect that the following errors would have on the calculatedpercent yield of the product. Would the yield be expected toincrease, decrease, or would there be no effect? Explainyour reasoning.– The product was insufficiently dried before weighing.– Some of the product was lost during the transfer fromthe Buchner funnel to the evaporating dish.– 7.5 mL of FeCl3 was added instead of 3.0 mL asoutlined in the procedure.– 4.587g of K2C2O4H2O was used instead of exactly4.000g .– The recrystallization step was skipped and theexperiment went straight to vacuum filtration.
- For the reduction 2FeCl3 + SnCl2 =====➔ 2 FeCl2 + SnCl4 in aqueous solution the following data were obtained at 25oC t(min) 1 3 7 11 40 Y 0.01434 0.02664 0.03612 0.04102 0.05058 Where y is the amount of FeCl3 reacted in moles per liter. The initial concentrations of SnCl3 and FeCl3 were respectively, 0.03125, 0.0625 moles/L. a.)Show that the reaction is third order (derive the rate law), and b.) calculate the average specific rate constant.. A solution was prepared by mixing 4.00mL of 2.00 x 10-3 M Fe(NO3)3 and 3.00mL of 5.00 x 10-3 M NaSCN and diluting the mixture with water to a total of 10.00mL. Use your average value of Kc to calculate the equilibrium concentration of FeSCN2+ in the mixture. [Hint: Use as many significant figures as you legitimately can in your calculations] my average you can find on the picture below PLS HELP ASAP!!8.00mL aqueous suspension of elemental selenium is treated with 24.00mL ammonia 0.045M AgNO3 The reaction is as follows: 6Ag (NH3) 2 ++ 3Se (s) + 3H2O → 2Ag2Se (s) + Ag2SeO3 (s) + 6NH4 + After this reaction is completed, nitric acid is added to dissolve Ag2SeO3. However, Ag2Se does not dissolve during this time. Ag + consisting of dissolved Ag2SeO3 and the excess of the reagent requires 13.43mL 0.01294M KSCN in a Volhard titration. How many milligrams of Se are in each milliliter of sample? (Se = 78.96g / mol)

