Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter12: Atomic X-ray Spectrometry
Section: Chapter Questions
Problem 12.6QAP
Related questions
Question
What is the working solution and DI water of the unknown solution?
Transcribed Image Text:power to electrical signals.
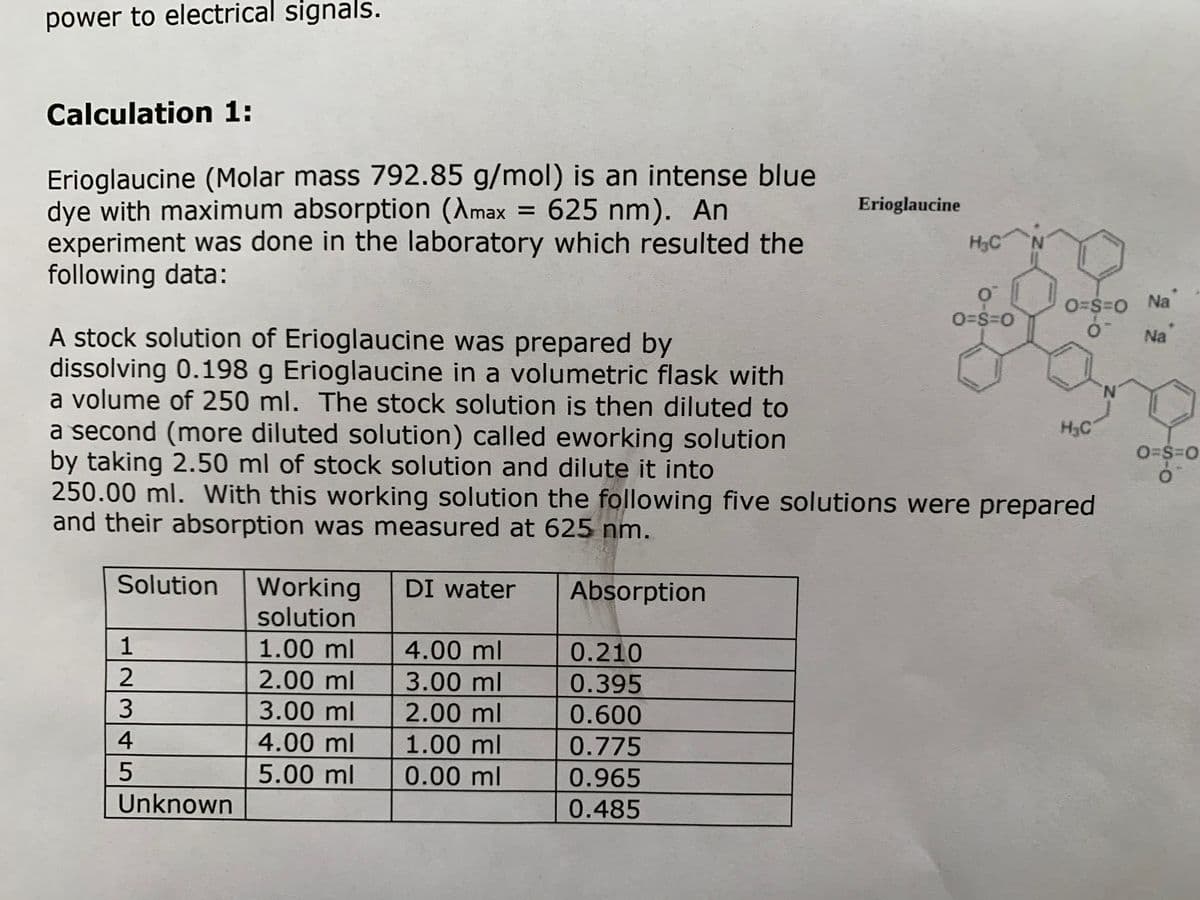
Calculation 1:
Erioglaucine (Molar mass 792.85 g/mol) is an intense blue
dye with maximum absorption (Amax = 625 nm). An
experiment was done in the laboratory which resulted the
following data:
%3D
Erioglaucine
H3C
N.
O=$=0 Na
O=S=0
A stock solution of Erioglaucine was prepared by
dissolving 0.198 g Erioglaucine in a volumetric flask with
a volume of 250 ml. The stock solution is then diluted to
a second (more diluted solution) called eworking solution
by taking 2.50 ml of stock solution and dilute it into
250.00 ml. With this working solution the following five solutions were prepared
and their absorption was measured at 625 nm.
Na
N.
H3C
Solution
Working
solution
DI water
Absorption
1.00 ml
4.00 ml
0.210
2.00 ml
3.00 ml
3.00 ml
0.395
2.00 ml
0.600
4.00 ml
1.00 ml
0.775
5.00 ml
0.00 ml
0.965
Unknown
0.485
12345
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole