-3.0 3.0 125 -20 48 -15 36 -1.0 42 -05 42 ignificantly, it that any DNA rose gel loading
- Explain how you were able to identify plasmid in each sample using the gel, considering how linear DNA fragments of different length are separated by agarose gel electrophoresis.
- Why do supercoiled, nicked and linear DNA sequences of the same size (kb) migrate at different rates during agarose gel electrophoresis?
- How does SYBR Safe® enable the visualisation of the location of DNA fragments on the gel?
Step by step
Solved in 3 steps

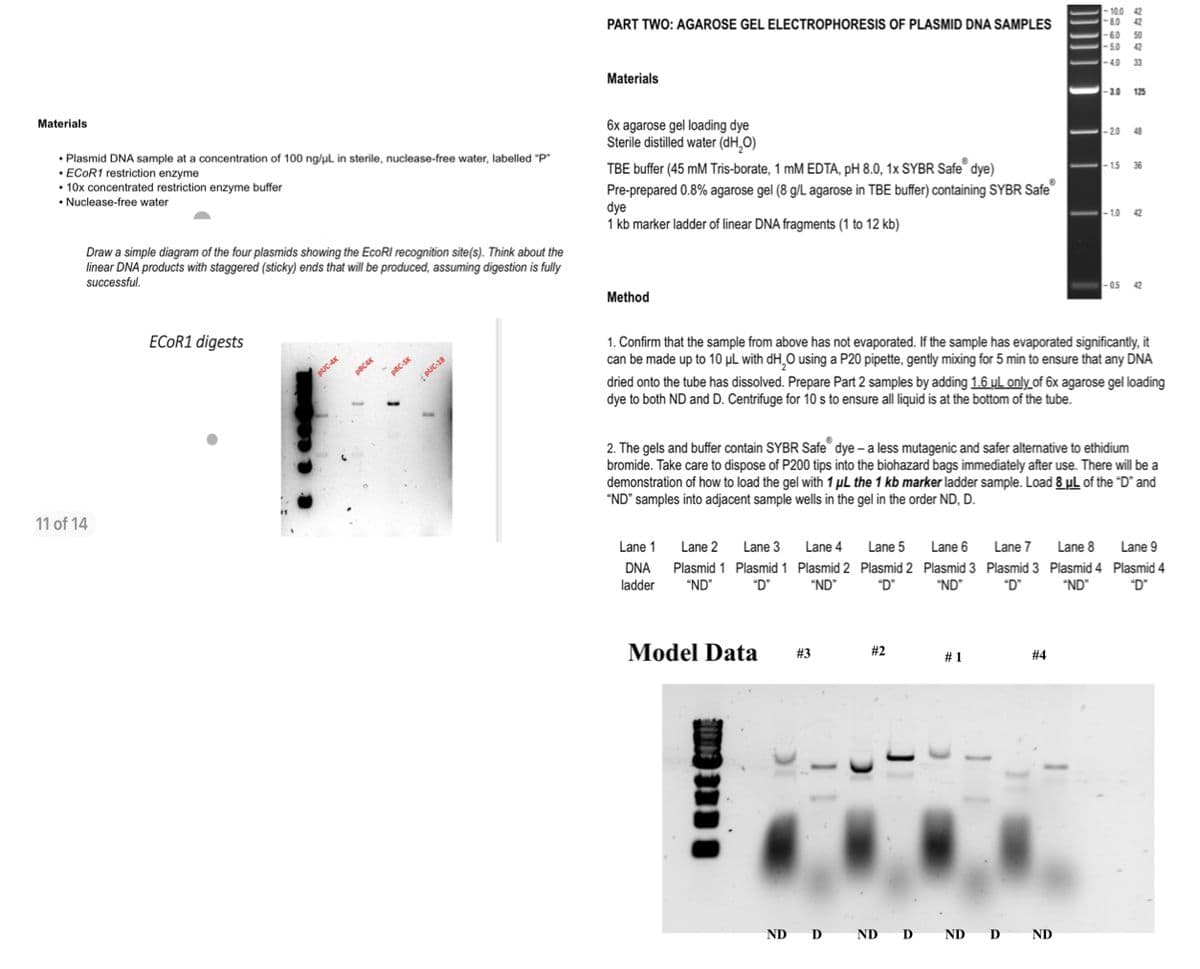
What legend would you give to the labeled model data?
Looking at the model data i upload and given the question how could i analyse tboth experimental results (antibiotic selection and agarose gel). Including one clearly labelled image of the agarose gel and with an accompanying legend. And how could i interpret the findings of the results and draw a conclusion i.e. what is in each tube? Also is there any further experiments that I could perform to confirm the identity of the plasmid stocks?








