What substance is obtained when the following separation method has been applied? Magnetic separation ____________________ Sublimation ___________________________ Filtration _____________________________ Evaporation__________________________
What substance is obtained when the following separation method has been applied? Magnetic separation ____________________ Sublimation ___________________________ Filtration _____________________________ Evaporation__________________________
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 13ALQ
Related questions
Question
What substance is obtained when the following separation method has been applied?
Magnetic separation ____________________
Sublimation ___________________________
Filtration _____________________________
Evaporation__________________________
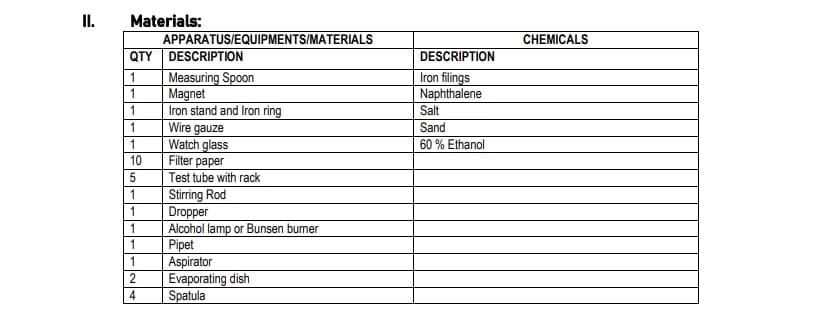
Transcribed Image Text:II.
Materials:
QTY DESCRIPTION
1
1
1
1
1
10
5
1
1
1
1
مان
1
APPARATUS/EQUIPMENTS/MATERIALS
2
4
Measuring Spoon
Magnet
Iron stand and Iron ring
Wire
gauze
Watch glass
Filter paper
Test tube with rack
Stirring Rod
Dropper
Alcohol lamp or Bunsen burner
Pipet
Aspirator
Evaporating dish
Spatula
DESCRIPTION
Iron filings
Naphthalene
Salt
Sand
60% Ethanol
CHEMICALS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole