Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 5CR
Related questions
Question
What would the balance reaction be for both of them?
Transcribed Image Text:* CO
MReview I Constants I Periodic
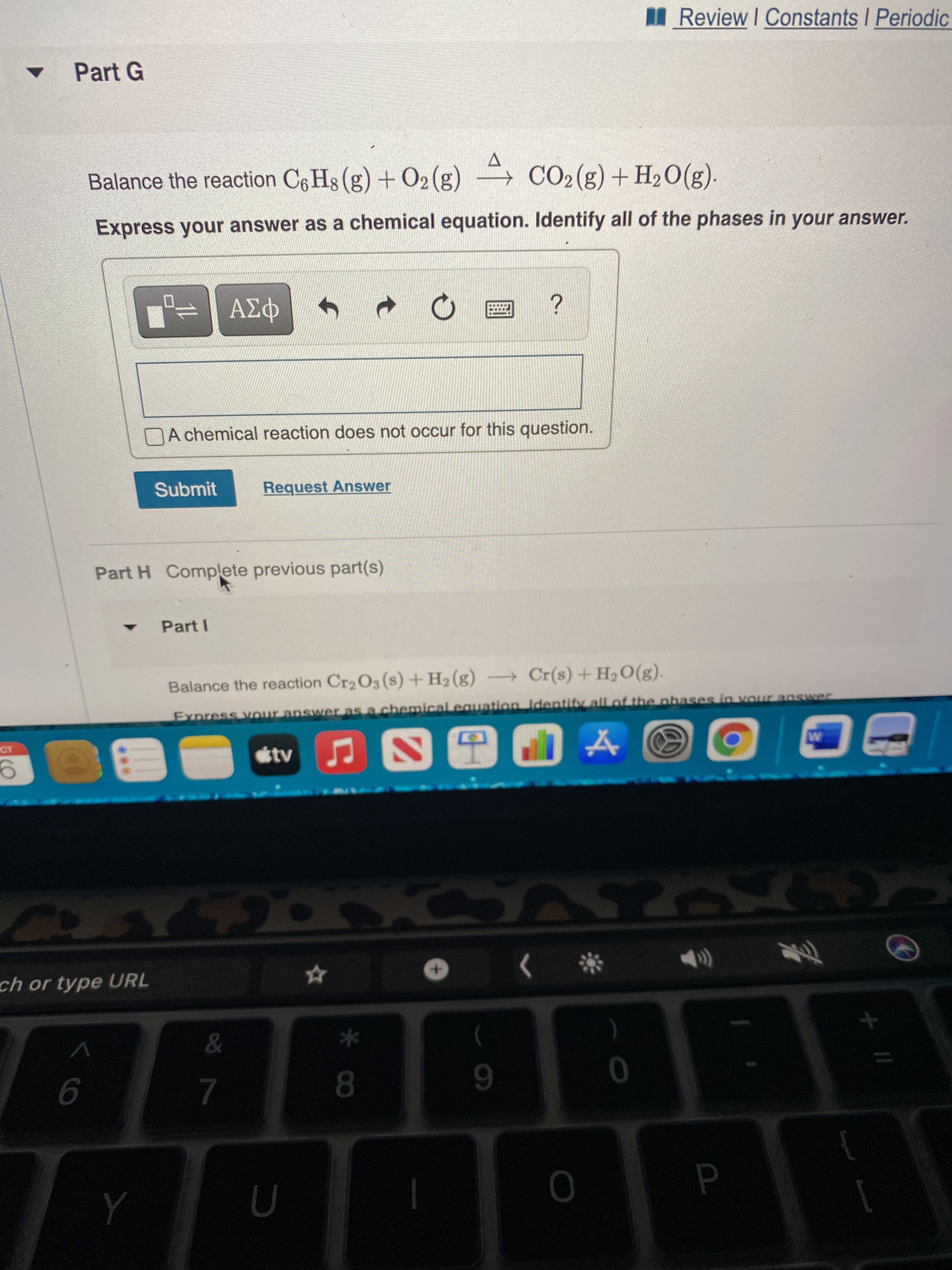
Part G
Balance the reaction C6 Hs (g) + O2(g) → CO2(g) + H2O(g).
Express your answer as a chemical equation. Identify all of the phases in your answer.
DA chemical reaction does not occur for this question.
Submit
Request Answer
Part H Complete previous part(s)
Part I
Balance the reaction Cr2O3 (s) +H2 (g) Cr(s) +H2O(g).
Express vour answer as a chemical equation Identifv all of the nhases in vour answer
ch or type URL
The
*
9.
8.
7.
Transcribed Image Text:* CO
< co
I Review I Constants I Periodic 7
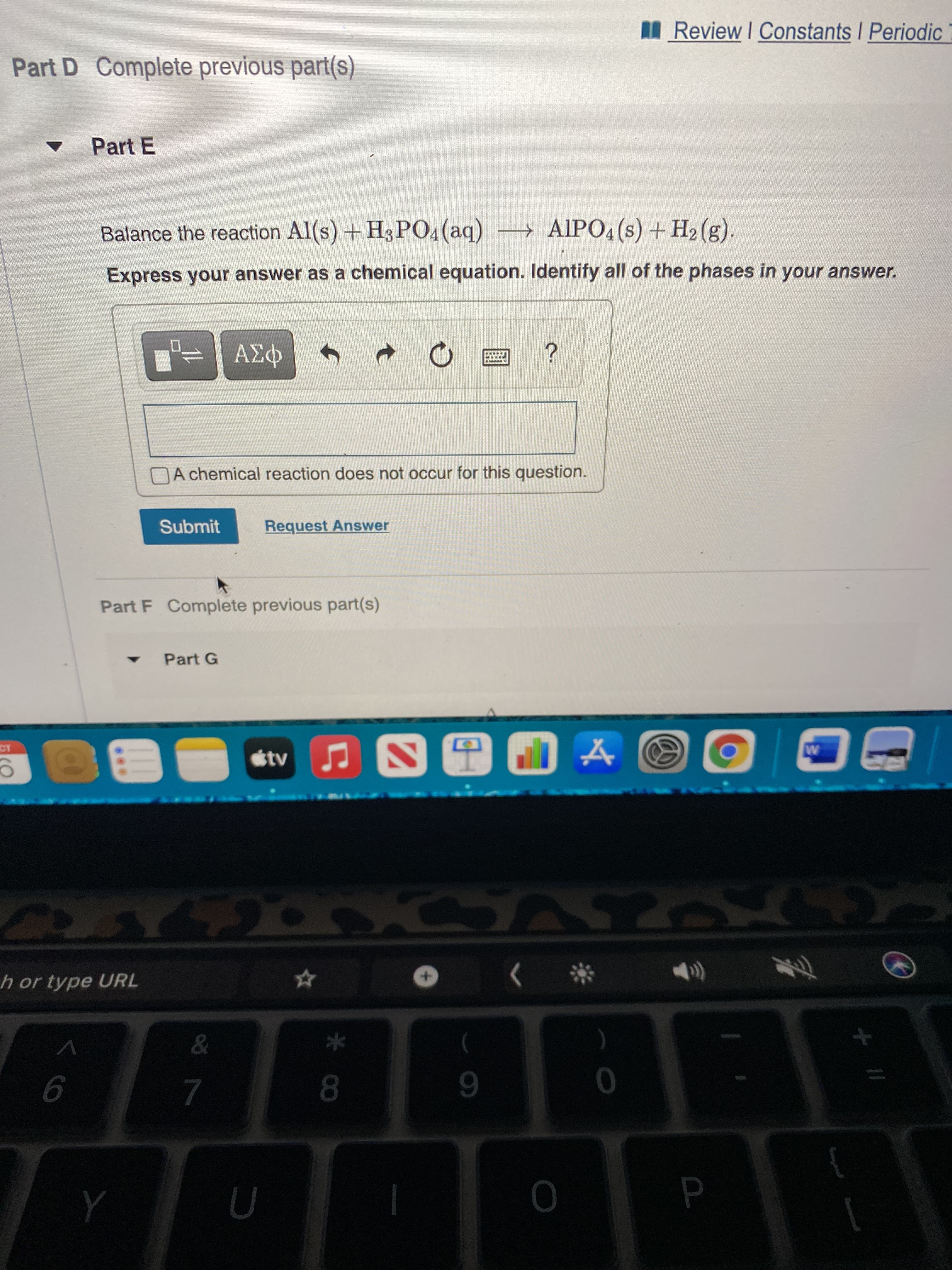
Part D Complete previous part(s)
Part E
Balance the reaction Al(s) +H3PO4 (aq) AIP04(s) + H2 (g).
Express your answer as a chemical equation. Identify all of the phases in your answer.
NA chemical reaction does not occur for this question.
Submit
Request Answer
Part F Complete previous part(s)
Part G
h or type URL
he
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning