Q: what is Faraday's first law of electrolysis ?
A: Electrolysis is the technique that involves the use of direct current for driving the chemical…
Q: Calculate the AG° for the following reaction at 25°C. You will have to look up the thermodynamic…
A:
Q: Define Galvanic Series.
A: Galvanic series has to be defined. The galvanic series is predominant for selection of material to…
Q: A common car battery consists of six identical cells, each of which carries out the reaction: Pb +…
A:
Q: Consider the following reaction at 298 K. I2(s) + H2(g) 2 I(aq) + 2 H*(aq) Which of the following…
A:
Q: 1. Use the calculation of E° to determine which of the following reaction is spontaneous under…
A: Electrode potential values linked with every half reaction are useful in calculation of cell…
Q: Calculate the standard free-energy change at 298K for the zinc-copper voltaic cell, which has a…
A:
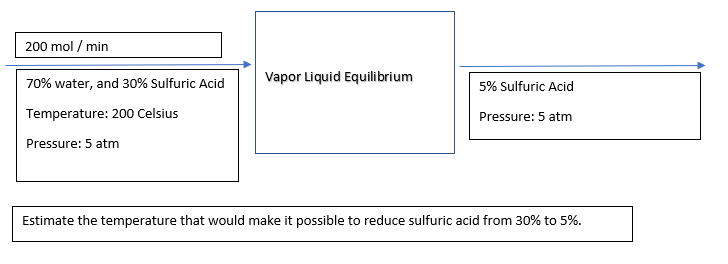
Q: Explain BALANCES ON REACTIVE PROCESSES?
A: Three different balances may be written to determine an unknown flow rate for a reactive process.…
Q: The mass of three different metal electrodes, each from a different galvanic cell, were determined…
A: The active electrode which is found to lose mass during redox reaction in a cell is termed as anode…
Q: state and explain Faraday’s laws of electrolysis. What is Electrochemicalequivalent?
A: Faraday’s first law of electrolysis states that the mass of the substance accumulated or released at…
Q: Convert 96750 Coulombs to Faradays.
A: A Faraday is a unit of charge. The given charge is = 96750 C The charge in Faraday is =?
Q: What unit is used to measure the magnitude of electrical current? What unit is used to measure the…
A: The unit used to measure the magnitude of electrical current is Amperes.The unit used to measure the…
Q: Calculate AG for the reaction at 298 K.
A: T=289K ∆H°=-32.8kJ ∆S°=-84.5J/K ∆G°=?
Q: Consider the following reaction at 298K. Hg2+ (aq) + Zn(s)→→→→→→ Hg({) + Zn²+ (aq) The standard…
A: The equation ∆G∘ = -nFE∘Cell can also be used to calculate the potential of half-cell by addition or…
Q: A strip of zinc metal immersed in a solution of a lead (II) salt will be oxidized, while the lead…
A: The standard reduction potential for a given cell refers to the tendency of a chemical species to…
Q: Define non-spontaneres process.
A: Thermo means heat and dynamic means motion. Together say heat in motion is what all studied in…
Q: Spontaneous reactions must occur under standard state conditions , yes or no
A: A reaction is categorized into two parts: spontaneous and non-spontaneous reactions. A spontaneous…
Q: Convert 61840 Coulombs to Faradays.
A:
Q: Explain the different parts of a galvanic cell and also include its functions.
A: A galvanic cell is a device in which chemical energy is converted into electrical energy as a result…
Q: Calculate the theoretical cell potential of the following cell. If the cell is short-circuited,…
A: The given cell notation is: Ag(s)|Ag+(aq)(0.0575 M) || H+(aq)(0.0333 M)| O2(g)(1.12 atm), Pt The…
Q: Calculate DG° f( in kJ) or the reaction of iron(II) ions with permanganate ions. (F =96500 C)…
A:
Q: The following reaction is __________ in the direction written under standard conditions at 25oC.…
A:
Q: Explain the following relationships: Delta G and w, cell potential and w, cell potential and Delta…
A: The equations for the relationship between Delta G and w, cell potential and w, cell potential and…
Q: Explain the concept of the Electrolysis of Water taking place at standard state. And why are the…
A: During electrolysis, electrical energy is used for the electrolysis. In an electrolytic cell two…
Q: Explain the Stoichiometry of Electrolysis?
A: The weights of substances formed at an electrode during electrolysis are directly proportional to…
Q: Will this reaction expected to be spontaneous as written? Assume standard conditions. Sn4+ + Fe --->…
A: The reaction given is Sn4+ + Fe -------> Sn2+ + Fe2+
Q: Calculate the standard free-energy change for the reaction at 25 ∘C.. Refer to the list of standard…
A: Standard reduction potential: E°Au+3/Au = 1.52 V E°Zn+2/Zn = 0.76 V Determine E° of the above…
Q: Fe (s) → Fe2+*(aq) + 2e¯, then Fe2*(aq) → Fe3*(aq) + e¯ (at anode) O2 (g) + 4H*(aq) + 4e → 2H2O(1)…
A:
Q: What happens to the chemical energy of a lithium-ion battery as it is discharged topower a laptop…
A: When the lithium ion battery is discharged to power a laptop it would produces the electrical…
Q: Briefly define steel corrosion. What are the four elements necessary for corro-sion to occur?
A: Corrosion : Corrosion is a natural process in which a metal is deteriorated or decayed by a chemical…
Q: Consider the following reaction at 298K. Pb2+ (aq) + 2 Cu+ (aq) → Pb (s) + 2 Cu²+ (aq) Which of the…
A: We have to predict the correct statement.
Q: standard reaction free energy AG
A:
Q: what is Faraday's second law of electrolysis ?
A: Electrolysis:Separation of a liquid into its chemical parts by passing direct electric current…
Q: determine whether each reaction is likely to occur spontaneously under standard conditions: a.…
A: First determine the E0value for given redox reaction. a. Sn(s) + Be2+(aq) → Sn2+(aq) + Be(s) Sn(s) +…
Q: What is the voltage of a galvanic cell that does 788 J of work when 255 Coulomb of charge is…
A:
Q: Calculate ∆Grxn for the oxidation of magnesium at 25°C
A: At constant temperature and pressure, the change in Gibbs free energy is defined as Δ G0 = Δ H0 − T…
Q: Is hydrogen nearly an ideal fuel?
A: Hydrogen how will be ideal fuel. Definition of ideal fuel -ideal fuel is a burning doesn't emit…
Q: b) determine the standard cell potential and if the cell is more or less spontaneous at the non…
A:
Q: Give the equilibrium constant for the following reaction at 298 K.
A: In the given cell reaction, cupric ion is being reduced to copper and Sn+2 is being oxidized to…
Q: Use the table below to write BALANCED, SPONTANEOUS, DIFFERENT reactions and determine their…
A:
Q: e standard reaction at 29
A:
Q: What is the function of a galvanic cell?
A: Electrochemical cells are the devices which convert chemical energy into electrical energy or also…
Q: Consider the following reaction at 298 K. Cr* (aq) + Fe2+ (aq) → Cr2+ (aq) + Fe+ (aq) Which of the…
A: Given rxn is non spontaneous, Since standard reduction potential value of Cr+2 is lesser than Fe+2…
Q: b) The image below shows the combustion of these three molecules. By examining the molar ratio each…
A: Combustion is the burning of fuel in the presence of oxygen. Combustion of organic substances yields…
Q: What is the standard Gibbs free energy change and equilibrium constant when Ag+ reacts with iron to…
A: Given: Ag+ reacts with iron to form iron(II) at 25oC. To answer: Writing a balanced chemical…
Q: What is the standard state of copper?
A: Standard state of an element is state /phase they exist at 25°C temperature and 1 atmoshperic…
Q: What type of reaction takes place in a galvanic cell? nonspontaneous spontaneous
A: A galvanic cell is an electrochemical cell that uses the transfer of electrons in redox reactions to…
Q: At what temperature Teq do the forward and reverse corrosion reactions occur in equilibrium?…
A: Gibbs energy equation is given by the formula- △G = △H -T△S ----------------(i) Where, △G…
Step by step
Solved in 2 steps

- A student obtains the following data by following an experimental procedure to this one. Based on this data, what is the value of R (in L.atm/mol/K) that would be calculated? Report the result to four significant figures. Use 273.15 K for the temperature conversion. Pressure of dry H2 = 742.0 mm Hg Temperature = 22.00 degrees C Mass of Mg used = 0.03860 g Volume of H2 = 44.10 ET units Conversion factor = 0.8119 mL/ET UnitYou have containers of pure O2 and N2 at 298 K and 1 atm pressure. You may assume the gases act like ideal gasses. Calculate ΔGmixing relative to the unmixed gases of: a) a mixture of 15 mol of O2 and 15 mol of N2. b) Calculate ΔGmixing if 12 mol of pure N2 are added to the mixture of 15 mol of N2 and 15 mol of O2. c) Calculate ΔSmixing for the system described in part b). d) Determine ΔHmixing for the system described in part b).Calculate the ΔHsoln and ΔSsoln for the dissolution of solid KHP from the trendline equation of your scatter graph. Include units. Reffer to equation 4. R is the gas constant 8.314J mol-1 K-1 The trendline equation -5623*x + 17.7 Equation 4 Ln Ksp = - ΔHsoln/R (1/T) + ΔSsoln/ R
- Use the molar DHf° under the formulas to calculate DH°rxn for the equations as balanced: 1. 2B2H6(g) + 3CO2(g) --> 2B2O3(s) + 3CH4(g) ΔH°rxn = _______ kJ ΔH°f = +36 –394 –1274 –75 kJ/mol exo ? endo_thermic 2. 2P2O5 + 2CaC2 -->P4 + 2CaCO3 + 2CO2 ΔH°rxn = _______ kJ ΔH°f = –1505 –59 ___ –1207 –394 kJ/mol exo ? endo_thermic 3. 2Na2CrO4(s) + 10HCl(g) --> 4NaCl(s) + 3Cl2(g) + Cr2O3(s) + 5H2O(l) ΔH°f = –1342 –92 –411 ___ –1140 –286 kJ/mol ΔH°rxn = _______ kJ exo ? endo_thermica. Consider the data below for the reaction H2O(l) ⇌ H2O(g) . Plot a graph and determine ΔH and ΔS for the reaction. Describe how they influence the spontaneity of the reaction as a function of temperature. T (°C) P (torr) 0.0 4.579 10.0 9.209 20.0 17.53 25.0 23.76 30.0 31.82 40.0 55.32 60.0 149.4 70.0 233.7 90.0 525.8 Explain how the boiling point temperature of H2O(l) (at sea level) can be accurately determined from the data in a.? c. For the reaction in a., ΔE is less than ΔH. Explain.Solve for the moIe fraction of methanoI in vapor phase assuming that the totaI pressure above its soIution with H2O is 80 torr at 20oC. Useful values: P*H2O=17.5 torr; P*methanol =97.7 torr
- A sample of gas in which [H2S] = 5.96 M is heated to 1400 K in a sealed vessel. After chemical equilibrium has been achieved, what is the value of [H2S]? Assume no H2 or S2 was present in the original sample.A sample of propane (C3H8)(C3H8) is placed in a closed vessel together with an amount of O2O2 that is 3.10 times the amount needed to completely oxidize the propane to CO2CO2 and H2OH2O at constant temperature. Calculate the mole fraction of each component in the resulting mixture after oxidation, assuming that the H2OH2O is present as a gas.Calculate ΔSvap for hydrogen sulfide from the following data: normal boiling point of H2S: -60.7oC ΔHvap of H2S: 18.7 kJ/mol Place ΔSvap in units of J/(mol K) and your answer should have 3 significant figures.
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 24.0cm wide and 28.8cm high. The maximum safe pressure inside the vessel has been measured to be 8.30MPa. For a certain reaction the vessel may contain up to 1.35kg of boron trifluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits.A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 53.0 cm wide and 63.6 cm high. The maximum safe pressure inside the vessel has been measured to be 7.40 MPa. For a certain reaction the vessel may contain up to 28.1 kg of sulfur hexafluoride gas, Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius, Round your answer to 3 significant digits.When 0.5 g of a liquid is completely evaporated and collected in a litermanometer, the pressure is 0.25 atm and the temperature is 27°C. Assumeideal gas behaviour, find the molecular weight if the gas constant is R =0.0821 atm/mole°K.

