When 25.0 g of AgNO, are reacted with excess NagPO4, 18.7 g of Ag PO, are pr duced. What is the percentage yield of Ag,PO4? 3 moles Ag NO2 I mole Ag 3PO4 3593D0,147moles of 69.87 91.29 AgiNOB gives O.049moles Aga Poy .047X418.589g Imol. -20159 307 mass mm. 18,7,180 = 91.2 2015 /00
When 25.0 g of AgNO, are reacted with excess NagPO4, 18.7 g of Ag PO, are pr duced. What is the percentage yield of Ag,PO4? 3 moles Ag NO2 I mole Ag 3PO4 3593D0,147moles of 69.87 91.29 AgiNOB gives O.049moles Aga Poy .047X418.589g Imol. -20159 307 mass mm. 18,7,180 = 91.2 2015 /00
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.39PAE: Methanol, CH3OH, is used in racing cars because it is a clean-burning fuel. It can be made by this...
Related questions
Question
I just need a step-by-step explanation for this problem I forgot how to Calculate percent yield
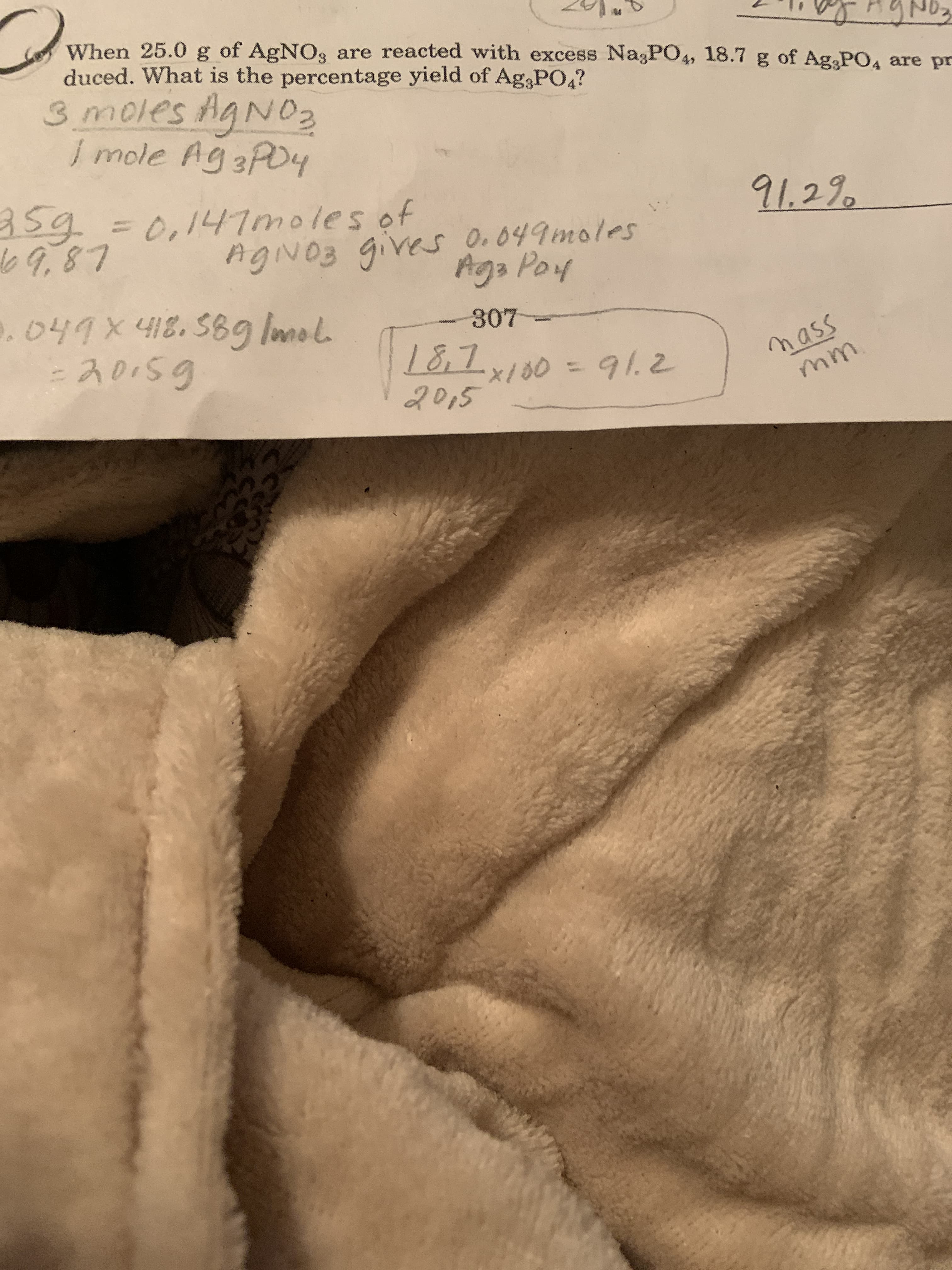
Transcribed Image Text:When 25.0 g of AgNO, are reacted with excess NagPO4, 18.7 g of Ag PO, are pr
duced. What is the percentage yield of Ag,PO4?
3 moles Ag NO2
I mole Ag 3PO4
3593D0,147moles of
69.87
91.29
AgiNOB gives O.049moles
Aga Poy
.047X418.589g Imol.
-20159
307
mass
mm.
18,7,180 = 91.2
2015
/00
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
