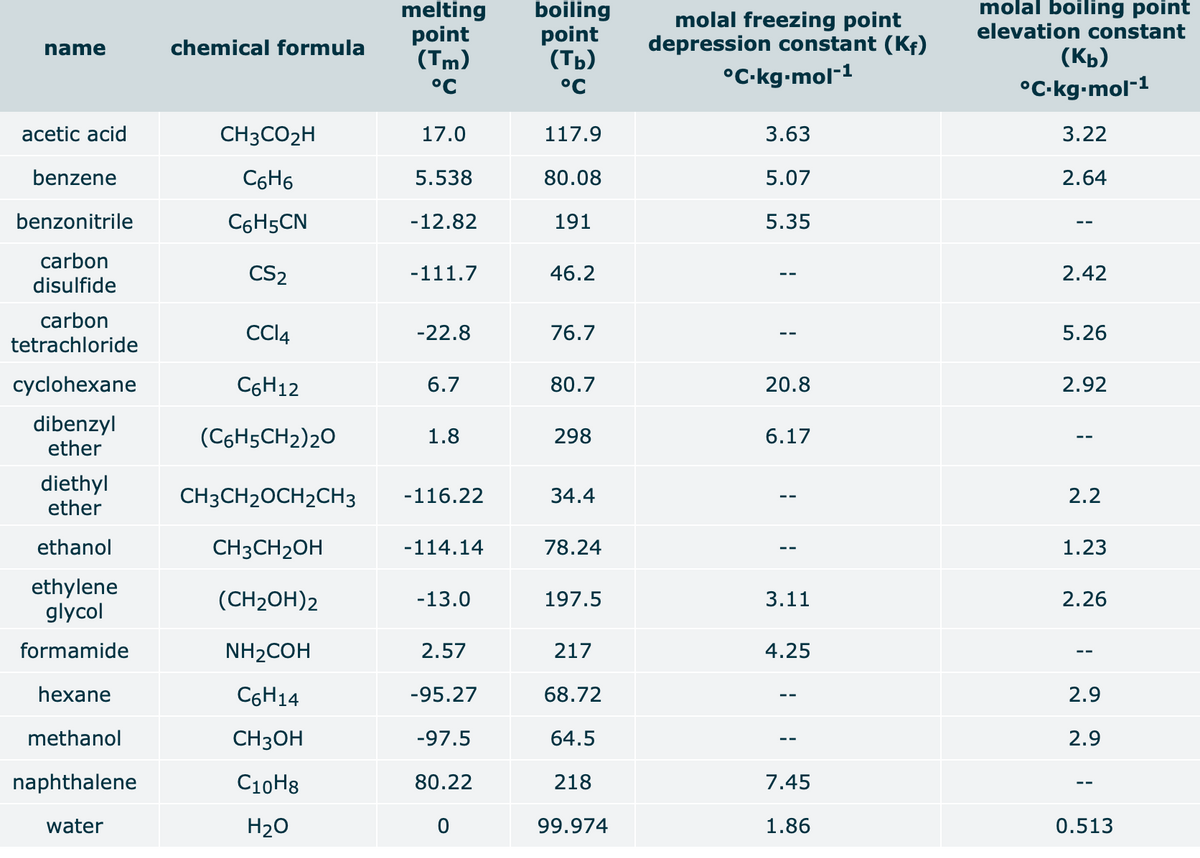
When 3.97 g of a certain molecular compound X are dissolved in 75.0 g of cyclohexane (C,H2), the freezing point of the solution is measured to be 4.7 °C. Calculate the molar mass of X. If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and rounded to the correct number of significant digits. Oxt0
When 3.97 g of a certain molecular compound X are dissolved in 75.0 g of cyclohexane (C,H2), the freezing point of the solution is measured to be 4.7 °C. Calculate the molar mass of X. If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and rounded to the correct number of significant digits. Oxt0
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 48QAP: The Rast method uses camphor (C10H16O) as a solvent for determining the molar mass of a compound....
Related questions
Question
100%

Transcribed Image Text:When 3.97 g of a certain molecular compound X are dissolved in 75.0 g of cyclohexane (C,H12), the freezing point of the solution is measured to be 4.7 °C.
Calculate the molar mass of X.
If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is
rounded to the correct number of significant digits.
x10
미□
Transcribed Image Text:molal boiling point
melting
point
(Tm)
boiling
point
(Ть)
°C
molal freezing point
depression constant (Kf)
elevation constant
name
chemical formula
(К»)
°C
°C-kg-mol-1
°C-kg-mol-1
acetic acid
CH3CO2H
17.0
117.9
3.63
3.22
benzene
C6H6
5.538
80.08
5.07
2.64
benzonitrile
C6H5CN
-12.82
191
5.35
carbon
CS2
-111.7
46.2
2.42
disulfide
carbon
CI4
-22.8
76.7
5.26
tetrachloride
cyclohexane
C6H12
6.7
80.7
20.8
2.92
dibenzyl
ether
(C6H5CH2)20
1.8
298
6.17
diethyl
ether
CH3CH20CH2CH3
-116.22
34.4
2.2
ethanol
CH3CH2OH
-114.14
78.24
1.23
ethylene
glycol
(CH2OH)2
-13.0
197.5
3.11
2.26
formamide
NH2COH
2.57
217
4.25
hexane
C6H14
-95.27
68.72
2.9
methanol
CH3OH
-97.5
64.5
2.9
naphthalene
C10H8
80.22
218
7.45
water
H20
99.974
1.86
0.513
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning