When 5.58 g of a solid mixture composed of NH4CI and CaCl2 was dissolved in 100.0 mL of water, the temperature of the water rose by 4.86°C. How many grams of each substance was in the mixture? Include the mass of salts in calculation of heat generated. g NH4CI = i g CaCl2 = i
When 5.58 g of a solid mixture composed of NH4CI and CaCl2 was dissolved in 100.0 mL of water, the temperature of the water rose by 4.86°C. How many grams of each substance was in the mixture? Include the mass of salts in calculation of heat generated. g NH4CI = i g CaCl2 = i
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter3: The Plasma Membrane And Membrane Potential
Section: Chapter Questions
Problem 1SQE: Using the Nernst equation, calculate the equilibrium potential for Ca2 and for C1 from the following...
Related questions
Question
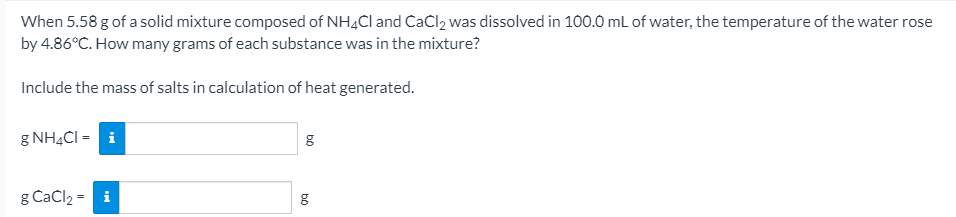
Transcribed Image Text:When 5.58 g of a solid mixture composed of NH4CI and CaCl2 was dissolved in 100.0 mL of water, the temperature of the water rose
by 4.86°C. How many grams of each substance was in the mixture?
Include the mass of salts in calculation of heat generated.
g NH4CI = i
g CaCl2 = i
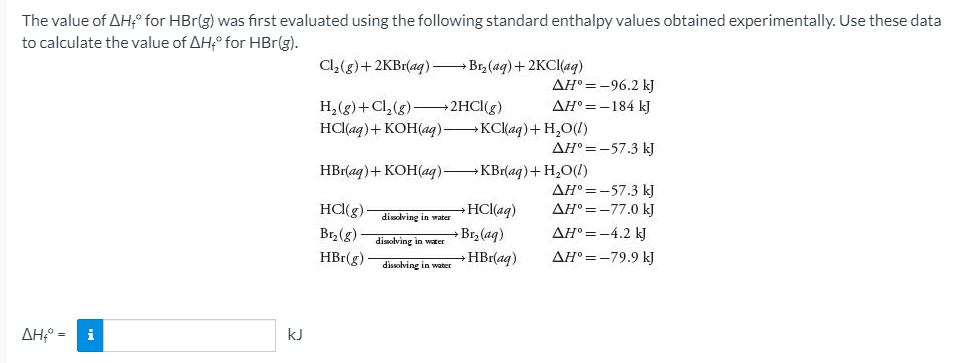
Transcribed Image Text:The value of AH° for HBr(g) was first evaluated using the following standard enthalpy values obtained experimentally. Use these data
to calculate the value of AHf° for HBr(g).
Cl,(g)+2KB1(ag) – Br, (ag)+2KCI(ag)
AH° =-96.2 kJ
H,(g)+Cl, (g)-
HCl(aq)+ KOH(aq) KC(aq)+H,O(!)
2HCI(g)
AH° =-184 kJ
AH° =-57.3 kJ
HBr(aq)+ KOH(aq) KBr(aq)+ H,O(!)
AH° =-57.3 kJ
AH° =-77.0 kJ
HC(g)
Br,(g)
HBr(g)-
HCl(aq)
disdlving in water
Br, (aq)
AH° =-4.2 kJ
disolving in water
HBr(aq)
AH° =-79.9 kJ
dissolving in water
ΔΗΡ-
i
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning


Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning


Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning
