When a metal bar is temporarily connected between a hot reservoir at Th and a cold reservoir at Tc, the energy transferred by heat from the hot reservoir to the cold reservoir is Qh. In this irreversible process, find expressions for the change in entropy of the following. (Use any variable or symbol stated above as necessary.) (a) the hot reservoir ΔSh = (b) the cold reservoir ΔSc = (c) the Universe ΔSU =
When a metal bar is temporarily connected between a hot reservoir at Th and a cold reservoir at Tc, the energy transferred by heat from the hot reservoir to the cold reservoir is Qh. In this irreversible process, find expressions for the change in entropy of the following. (Use any variable or symbol stated above as necessary.) (a) the hot reservoir ΔSh = (b) the cold reservoir ΔSc = (c) the Universe ΔSU =
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter18: Heat Engines, Entropy, And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 34P
Related questions
Question
When a metal bar is temporarily connected between a hot reservoir at Th and a cold reservoir at Tc, the energy transferred by heat from the hot reservoir to the cold reservoir is Qh. In this irreversible process, find expressions for the change in entropy of the following. (Use any variable or symbol stated above as necessary.)
(a) the hot reservoir
ΔSh =
(b) the cold reservoir
ΔSc =
(c) the Universe
ΔSU =
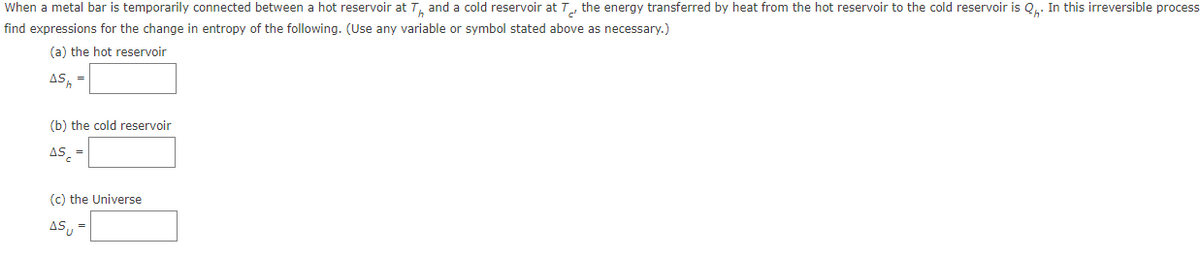
Transcribed Image Text:When a metal bar is temporarily connected between a hot reservoir at T, and a cold reservoir at T, the energy transferred by heat from the hot reservoir to the cold reservoir is Q.. In this irreversible process
find expressions for the change in entropy of the following. (Use any variable or symbol stated above as necessary.)
(a) the hot reservoir
AS, =
(b) the cold reservoir
AS =
(c) the Universe
AS, =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning
