When a solid dissolves in water, hest may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 5.99 g of NH, Br(s) are dissolved in 117.40 g of water, the temperature of the solution drops from 25.98 to 23.69 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.75 J/"C. Based on the student's observation, calculate the enthalpy of dissolution of NH,Br(s) in kJ/mol. Assume the specific heat of the solution is equal to the specific heat of water. AHaisolution kJ/mol
When a solid dissolves in water, hest may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 5.99 g of NH, Br(s) are dissolved in 117.40 g of water, the temperature of the solution drops from 25.98 to 23.69 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.75 J/"C. Based on the student's observation, calculate the enthalpy of dissolution of NH,Br(s) in kJ/mol. Assume the specific heat of the solution is equal to the specific heat of water. AHaisolution kJ/mol
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.144QP: A piece of iron was heated to 95.4C and dropped into a constant-pressure calorimeter containing 284...
Related questions
Question

Transcribed Image Text:When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be
determined using a coffee cup calorimeter.
Thermo
In the laboratory a general chemistry student finds that when 5.99 g of NH,Br(s) are dissolved in 117,40 g of
water, the temperature of the solution drops from 25.98 to 23.69 C.
The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a
separate experiment to be 1.75 J/"C,
Based on the student's observation, calculate the enthalpy of dissolution of NH Br(s) in kJ/mol.
Assume the specific heat of the solution is equal to the specific heat of water.
AHaissohtion
kJ/mol
Submit Answer
Retry Entire Group
3 more group attempts remaining
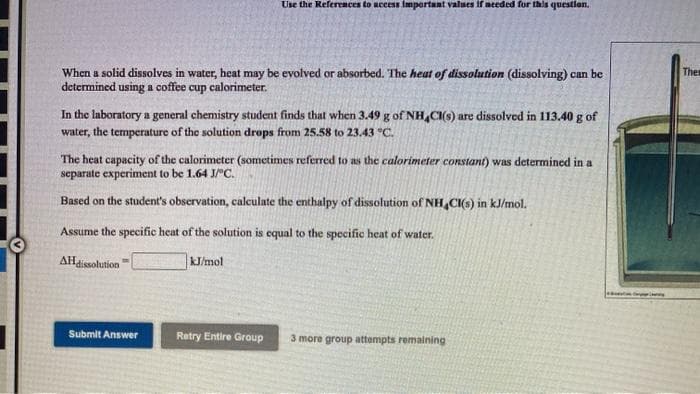
Transcribed Image Text:Use the References to uccess important values if needed for this question.
When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be
determined using a coffee cup calorimeter.
Thes
In the laboratory a general chemistry student finds that when 3.49 g of NH,CI(s) are dissolved in 113.40 g of
water, the temperature of the solution drops from 25.58 to 23.43 °C.
The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a
separate experiment to be 1.64 J/"C.
Based on the student's observation, calculate the enthalpy of dissolution of NH CI(s) in kJ/mol.
Assume the specific heat of the solution is equal to the specific heat of water.
AHisolution
kJ/mol
Submit Answer
Retry Entire Group
3 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax