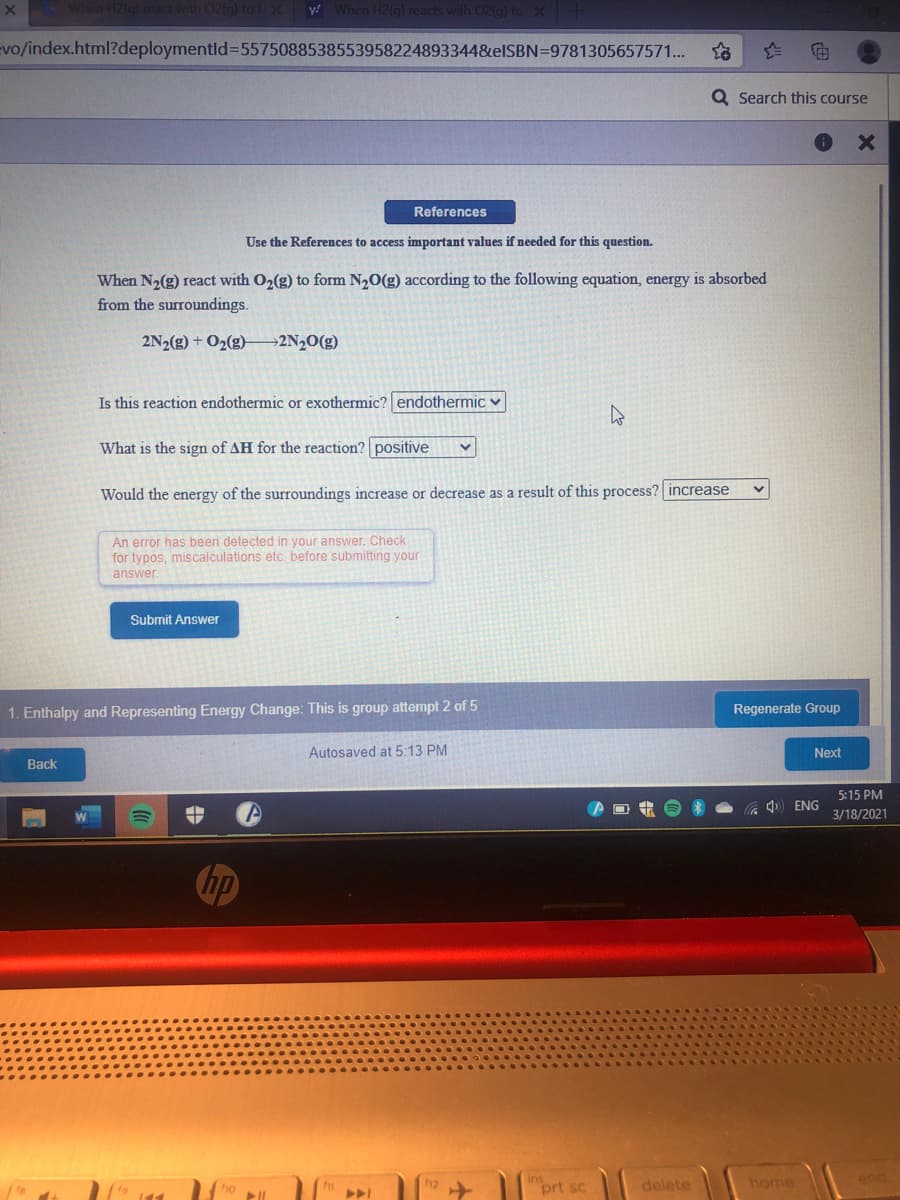
When N2(g) react with O2(g) to form N20(g) according to the following equation, energy is absorbed from the surroundings. 2N2(g) + O2(g)→2N2O(g) Is this reaction endothermic or exothermic? endothermic v What is the sign of AH for the reaction? positive Would the energy of the surroundings increase or decrease as a result of this process? increase
When N2(g) react with O2(g) to form N20(g) according to the following equation, energy is absorbed from the surroundings. 2N2(g) + O2(g)→2N2O(g) Is this reaction endothermic or exothermic? endothermic v What is the sign of AH for the reaction? positive Would the energy of the surroundings increase or decrease as a result of this process? increase
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 18E
Related questions
Question
Transcribed Image Text:1210)react with O2G) to i X
y! When H2g) reacts with 02
Evo/index.html?deploymentld%3D55750885385539582248933448&EISBN=9781305657571...
Q Search this course
References
Use the References to access important values if needed for this question.
When N2(g) react with O2(g) to form N,0(g) according to the following equation, energy is absorbed
from the surroundings.
2N2(g) + O2(g)–→2N½O(g)
Is this reaction endothermic or exothermic? endothermic v
What is the sign of AH for the reaction? positive
Would the energy of the surroundings increase or decrease as a result of this process? increase
An error has been detected in your answer. Check
for typos, miscalculations etc. before submitting your
answer.
Submit Answer
1. Enthalpy and Representing Energy Change: This is group attempt 2 of 5
Regenerate Group
Autosaved at 5:13 PM
Next
Back
5:15 PM
C ENG
3/18/2021
hp
ins
prt sc
delete
end
home
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning