When substances form liquid solutions, what two factors are involved in determining the solubility of the solute in the solvent? O The tendency towards randomness drives the solution process. O The new solvent-solute intermolecular attractions must be much weaker than the solvent-solvent and solute-solute attractions. O The tendency towards order drives the solution process. O The new solvent-solute intermolecular attractions must be about the same as the solvent-solvent and solute-solute attractions.
When substances form liquid solutions, what two factors are involved in determining the solubility of the solute in the solvent? O The tendency towards randomness drives the solution process. O The new solvent-solute intermolecular attractions must be much weaker than the solvent-solvent and solute-solute attractions. O The tendency towards order drives the solution process. O The new solvent-solute intermolecular attractions must be about the same as the solvent-solvent and solute-solute attractions.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.115QP: Two samples of sodium chloride solutions are brought to a boil on a stove. One of the solutions...
Related questions
Question
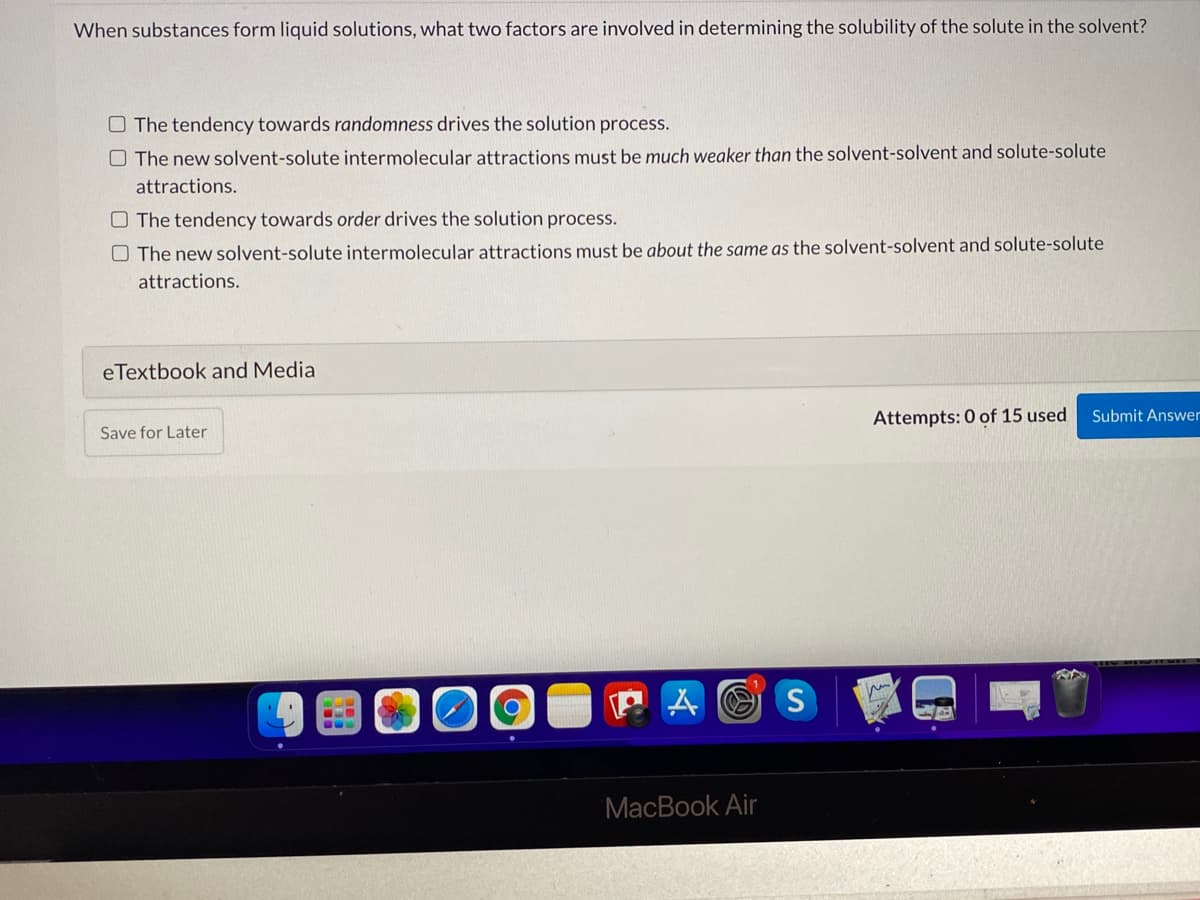
Transcribed Image Text:When substances form liquid solutions, what two factors are involved in determining the solubility of the solute in the solvent?
O The tendency towards randomness drives the solution process.
O The new solvent-solute intermolecular attractions must be much weaker than the solvent-solvent and solute-solute
attractions.
O The tendency towards order drives the solution process.
O The new solvent-solute intermolecular attractions must be about the same as the solvent-solvent and solute-solute
attractions.
eTextbook and Media
Attempts: 0 of 15 used
Submit Answer
Save for Later
P A O S
МacВook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning