When you open a bottle of soda, bubbles rapidly form and gas fizzes out. What is the best explanation of this? When the bottle is opened, the pressure of CO2 gas above the liquid rapidly drops, which significantly decreases the solubility of CO, in the soda, causing it to bubble out. The act of opening the bottle 'dislodges' tiny, nearly invisible gas bubbles that were clinging to the side of the container. They quickly condense into larger bubbles and escape. H2CO3 in the soda reacts with water vapor in the air, forming CO2 that quickly bubbles out. Gas that was trapped at the top of the bottle between the cap and the surface of the liquid escapes immediately and 'draws' liquid soda as it escapes, creating the illusion of bubbling. Warm air from outside the bottle heats the soda, decreasing the solubility of the CO2 in the soda and causing it to bubble out. estion 7
When you open a bottle of soda, bubbles rapidly form and gas fizzes out. What is the best explanation of this? When the bottle is opened, the pressure of CO2 gas above the liquid rapidly drops, which significantly decreases the solubility of CO, in the soda, causing it to bubble out. The act of opening the bottle 'dislodges' tiny, nearly invisible gas bubbles that were clinging to the side of the container. They quickly condense into larger bubbles and escape. H2CO3 in the soda reacts with water vapor in the air, forming CO2 that quickly bubbles out. Gas that was trapped at the top of the bottle between the cap and the surface of the liquid escapes immediately and 'draws' liquid soda as it escapes, creating the illusion of bubbling. Warm air from outside the bottle heats the soda, decreasing the solubility of the CO2 in the soda and causing it to bubble out. estion 7
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 57GQ: Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a reagent to precipitate nickel ion. Assume that 53.0...
Related questions
Question
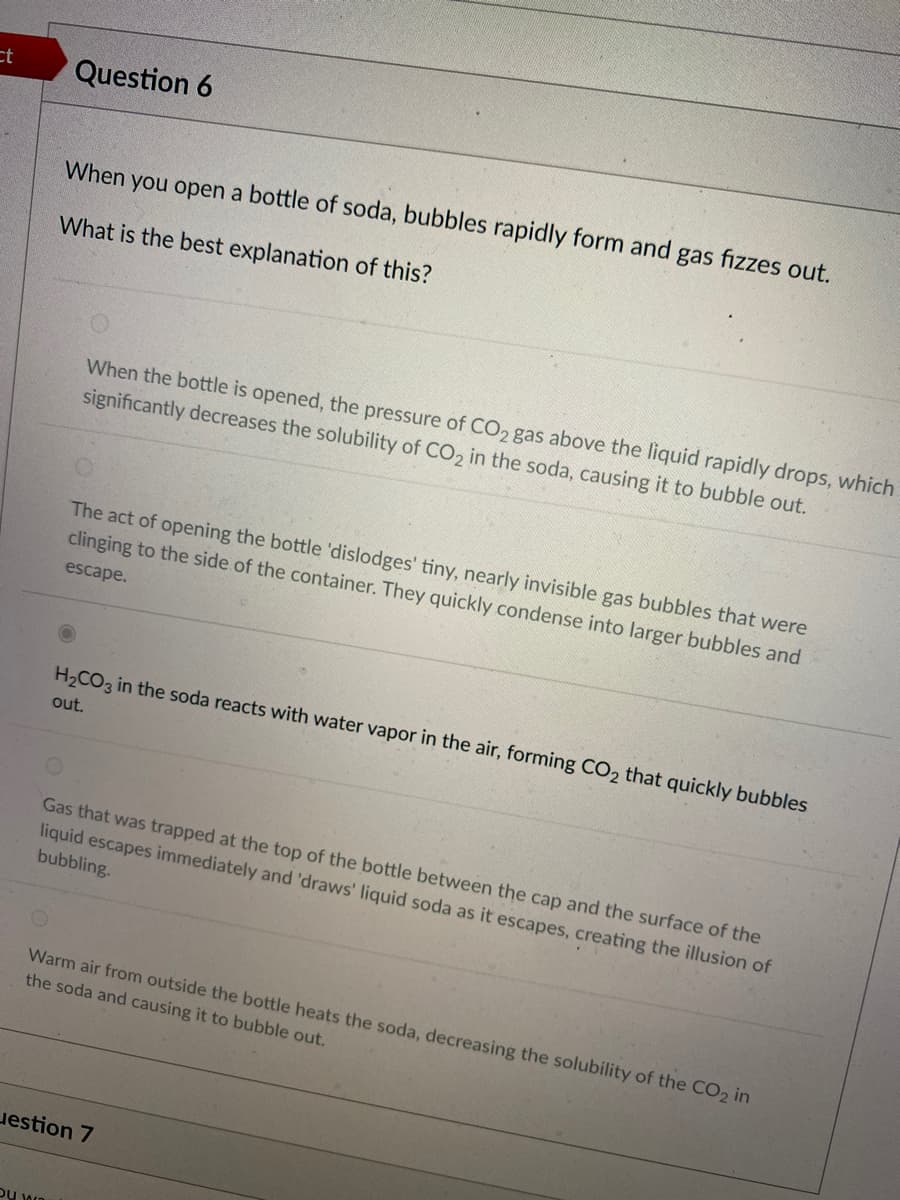
Transcribed Image Text:ct
Question 6
When you open a bottle of soda, bubbles rapidly form and gas fizzes out.
What is the best explanation of this?
When the bottle is opened, the pressure of CO2 gas above the liquid rapidly drops, which
significantly decreases the solubility of CO, in the soda, causing it to bubble out.
The act of opening the bottle 'dislodges' tiny, nearly invisible gas bubbles that were
clinging to the side of the container. They quickly condense into larger bubbles and
escape.
H2CO3 in the soda reacts with water vapor in the air, forming CO2 that quickly bubbles
out.
Gas that was trapped at the top of the bottle between the cap and the surface of the
liquid escapes immediately and 'draws' liquid soda as it escapes, creating the illusion of
bubbling.
Warm air from outside the bottle heats the soda, decreasing the solubility of the CO2 in
the soda and causing it to bubble out.
uestion 7
Du wa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning