Where do you expect to find elements that are good oxidizing agents on the periodic table? Periodic Table of the Elements LA 11A H 1.00 LI Unte 6.941 37 K 30.090 12 Na Mg Seder Magn 24,305 Rb 54.486 A Be BeryBum 9.012 B 2 ILA RA 2A 20 D Ca 40.078 38 56 Cs Ba Osem Datum Fr Ra Frandlem Podium 223.020 220.025 Sr Strontin 67.62 21 Sc Sondum 44 558 39 3 118 30 Y Yorum 88.900 57-71 Lanthanide Series Actinide Series 22 23 TI V Thark 47.88 40 4 IVB 401 72 Hf Than 178.40 Zr Zroonkam Nobium 91.224 92.906 Lantanum 138.906 Ac belician 90 5 VD 50 BRAS 50.942 La Ce Cartur Altai Metal 73 at the bottom 104 Rf Rutherfordium Dubnium Seaborgum 24 41 42 43 Nb Mo Tc Molybdunum Technetum 95.94 98.907 75 6 VID 60 25 26 Cr Mn Fe Commem Ch 51.000 Iron 55.833 on the left side 74 Ta W Tantalum Borgen 180 945 18585 7 VIID 70 Alkaline Earth Manganese 54335 105 106 Db Sg Bh Bellum 264 C in the transition metals Th Pa U Thoilure Prousticien Uhanikum 232.038 221.030 Re. Os Thanam 108 207 Ourum 190.23 107 44 Transition Metal 108 27 Hs Hecskem (2001 VIII Co 58.833 59 50 61 62 Pr Nd Pm Sm Prem Neymum 140 FOR 144.24 Promotiom 144 913 150.36 Ir Indum 192 22 109 45 46 47 48 49 Rh Tc Ru Rh Pd Ag Cd In Ru Calrike 112411 Rutenium Rodium Puudus 101.07 102.900 106.42 76 Shar 107.808 79 Indum 114.318 81 77 10 63 28 29 30 Ni Cu Zn ZINE 4539 Na 58.003 Semimetal Nonmetal Mt Meu Demon Ron [268] 24 195.00 Copper 83548 on the far right side (except for the noble gases) Eu Cadabrim Fungim 181.906 157.25 Bask Metal 12 In 201 22 13 Halogen B en 10.811 13 RA 3A Al Marines 21.982 31 6 Noble Gas 14 NA 4A C Cartre 12.011 14 32 Ga Ge Coffee Germanum 89.732 72.81 30 N 14.007 15 Si P S Som 28.06 Phosphona 30.974 Sulfa 32.000 The 118.71 15 VA SA 33 As Amark 74 822 51 68 16 VIA BA 16 S 34 O F Fluore 16.908 Lanthanide Actinide on 64 65 55 67 Gd Tb Dy Ho Tem Dysprofum Holmum 106.825 182.50 154 330 97 98 99 95 96 100 102 Fm Md No Neptatum Plutorium Arturicium Calum Berkan Culfanium Shum Furfum Murdolofum Noviem 237 048 244.064 243.001 247,070 247.070 251.000 (254) 257.096 Np Pu Am Cm Bk Cf Es 258.1 254.100 Se Seben 78.09 32 2 17 He VIA Heban 7A 17 Sn Sb Te 1 1276 Reimary ToBurlure 121.700 83 lodine 121.904 85 78 80 82 84 Pt Au Hg Tl Pb Bi Po At Load Puleniumm Gold 19967 111 Mercury 2004 112 204.383 113 2012 114 20140 200 115 116 200087 117 118 110 Ds Rg Cn Uut FI Uup Lv Uus Uuo Capemirs Uhrerlum PM Ferskum Dhunperdu Uemotum Unseptin Chunorius 280 (296) unknown unknown 70 Er Tm Yb Dulu Ditam 167.26 Yer 173.04 140 334 101 CI 35.453 Br Sunter 75 904 53 18 VITA BA 10 71 Ne Nen 20.100 18 Ar Fran 20.048 36 Kr Krypter 8436 54 Xe Saver 131.29 86 Rn Re 202.018 Lu 174.347 100 Lr COME
Where do you expect to find elements that are good oxidizing agents on the periodic table? Periodic Table of the Elements LA 11A H 1.00 LI Unte 6.941 37 K 30.090 12 Na Mg Seder Magn 24,305 Rb 54.486 A Be BeryBum 9.012 B 2 ILA RA 2A 20 D Ca 40.078 38 56 Cs Ba Osem Datum Fr Ra Frandlem Podium 223.020 220.025 Sr Strontin 67.62 21 Sc Sondum 44 558 39 3 118 30 Y Yorum 88.900 57-71 Lanthanide Series Actinide Series 22 23 TI V Thark 47.88 40 4 IVB 401 72 Hf Than 178.40 Zr Zroonkam Nobium 91.224 92.906 Lantanum 138.906 Ac belician 90 5 VD 50 BRAS 50.942 La Ce Cartur Altai Metal 73 at the bottom 104 Rf Rutherfordium Dubnium Seaborgum 24 41 42 43 Nb Mo Tc Molybdunum Technetum 95.94 98.907 75 6 VID 60 25 26 Cr Mn Fe Commem Ch 51.000 Iron 55.833 on the left side 74 Ta W Tantalum Borgen 180 945 18585 7 VIID 70 Alkaline Earth Manganese 54335 105 106 Db Sg Bh Bellum 264 C in the transition metals Th Pa U Thoilure Prousticien Uhanikum 232.038 221.030 Re. Os Thanam 108 207 Ourum 190.23 107 44 Transition Metal 108 27 Hs Hecskem (2001 VIII Co 58.833 59 50 61 62 Pr Nd Pm Sm Prem Neymum 140 FOR 144.24 Promotiom 144 913 150.36 Ir Indum 192 22 109 45 46 47 48 49 Rh Tc Ru Rh Pd Ag Cd In Ru Calrike 112411 Rutenium Rodium Puudus 101.07 102.900 106.42 76 Shar 107.808 79 Indum 114.318 81 77 10 63 28 29 30 Ni Cu Zn ZINE 4539 Na 58.003 Semimetal Nonmetal Mt Meu Demon Ron [268] 24 195.00 Copper 83548 on the far right side (except for the noble gases) Eu Cadabrim Fungim 181.906 157.25 Bask Metal 12 In 201 22 13 Halogen B en 10.811 13 RA 3A Al Marines 21.982 31 6 Noble Gas 14 NA 4A C Cartre 12.011 14 32 Ga Ge Coffee Germanum 89.732 72.81 30 N 14.007 15 Si P S Som 28.06 Phosphona 30.974 Sulfa 32.000 The 118.71 15 VA SA 33 As Amark 74 822 51 68 16 VIA BA 16 S 34 O F Fluore 16.908 Lanthanide Actinide on 64 65 55 67 Gd Tb Dy Ho Tem Dysprofum Holmum 106.825 182.50 154 330 97 98 99 95 96 100 102 Fm Md No Neptatum Plutorium Arturicium Calum Berkan Culfanium Shum Furfum Murdolofum Noviem 237 048 244.064 243.001 247,070 247.070 251.000 (254) 257.096 Np Pu Am Cm Bk Cf Es 258.1 254.100 Se Seben 78.09 32 2 17 He VIA Heban 7A 17 Sn Sb Te 1 1276 Reimary ToBurlure 121.700 83 lodine 121.904 85 78 80 82 84 Pt Au Hg Tl Pb Bi Po At Load Puleniumm Gold 19967 111 Mercury 2004 112 204.383 113 2012 114 20140 200 115 116 200087 117 118 110 Ds Rg Cn Uut FI Uup Lv Uus Uuo Capemirs Uhrerlum PM Ferskum Dhunperdu Uemotum Unseptin Chunorius 280 (296) unknown unknown 70 Er Tm Yb Dulu Ditam 167.26 Yer 173.04 140 334 101 CI 35.453 Br Sunter 75 904 53 18 VITA BA 10 71 Ne Nen 20.100 18 Ar Fran 20.048 36 Kr Krypter 8436 54 Xe Saver 131.29 86 Rn Re 202.018 Lu 174.347 100 Lr COME
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.122QP: A constant current of 1.25 amp is passed through an electrolytic cell containing a 0.050 M solution...
Related questions
Question
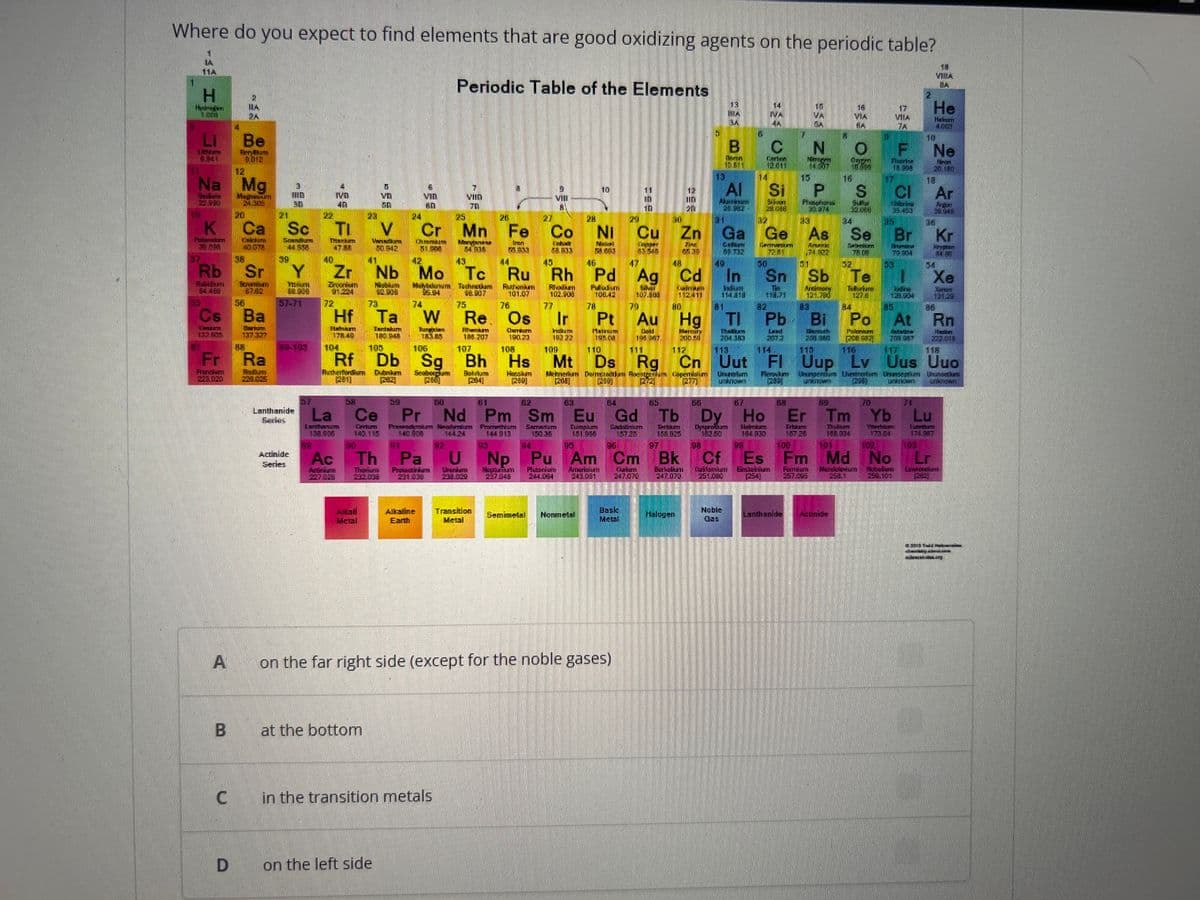
Transcribed Image Text:Where do you expect to find elements that are good oxidizing agents on the periodic table?
H
Hyding wi
LI
Na Mg
Rb
A
B
Fr Ra
ANITA
C
na
20
D
BOOTGAN
BO
Coandiumi
Series
TUNIT
58003
Lanthanide
IND
TI
Tresckum
1788
Zr
ZACHO
e
HAFGRATI
IPNJA
Rf
Rutherfordum
200
Lanthanum
158 800
Ac
Actiturn
La Ce
Cerium
140.115
T
160
Verndum
at the bottom
Nb
on the left side
VID
Hf Ta W Re. Os
tanislum
Jurging
ONTRUIT
59
Periodic Table of the Elements
24
Cr Mn Fe
thromium
Ursin
384
VID
IND
Mo
Mo Tc
Th Pa U
232.038
Db Sg Bh Hs
Scoberjum Bahrium
Hossum
in the transition metals
MA
Ru
Ru
ROUKHIRD
101.07
Transition
Metal
15
NII
8
FERIN
Co
NE
SENERE
RIKKET
102.000
10
Ir
Indrum
18722
NI
Nicht
2001
Pt
MU
AROW
16
10
Rh Pd Ag Cd
Rh
SINUT
KROKO
PI
106.12
112411
132
Pr Nd Pm Sm Eu Gd
Fraseodymium Neodymium
Samanum
150.36
on the far right side (except for the noble gases)
HO
Cu
12.
Back
Matal
555
Au
Gold
193 687
109
110
Mt Ds
Ds Rg
1157 25
Np Pu Am Cm
Nepanum Plutonium
237.048 241001
LITH
243.081
Zn
S
Hg
MERCURY
POOR
#15
B C
KUMMIK
Hom
DOUBL
Noble
COS
AI
FAHMI
UNTURI
In
THAIGHS
***
Si
Sn
Ge As
Hermanium
Amark
Palma
MAISONNI
ZA
** o * 8
Cf
Es
Surinam Ganun
1251
B:
Sb Te
1210000
1010
Tb Dy Ho Er Tm
15HIDR
171 030
Tm Yb
Bi Po At
101
Fm Md
*** -
Md No
14581
19005
Lanthanide Actinide
He
HIFTEI
Rg Cn Uut Fl Uup Lv Uus Uuo
Ununporium
Chupkemakm
||(1203)
Nuw
VIIIA
Hahum
4000
Ne
70 190
CI Ar
ESPON
Jobus
3
Br Kr
2
Xe
Xenon
131.29
Rn
Ilsdon
Lr
TWEVERSU
12241
Lu
Lustum
17107
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning