Which acid would be the best to combine with its sodium salt to make a solution buffered at pH 5.25? If you had 500.0 mL of a 0.10 M solution of the acid, what mass of the corresponding sodium salt of the conjugate base would you need to make the buffer?
Which acid would be the best to combine with its sodium salt to make a solution buffered at pH 5.25? If you had 500.0 mL of a 0.10 M solution of the acid, what mass of the corresponding sodium salt of the conjugate base would you need to make the buffer?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.21QAP
Related questions
Question
Which acid would be the best to combine with its sodium salt to make a solution
buffered at pH 5.25? If you had 500.0 mL of a 0.10 M solution of the acid, what
mass of the corresponding sodium salt of the conjugate base would you need to
make the buffer?
Transcribed Image Text:f Facebook
+ @ ☎ - B
X A GENERAL CHEMISTRY 2 - GX
Page not found - Chemistry X
(5) 5.00 g NaOH and 5.00 g x
Success Confirmation of Qu X
holodeck
drive.google.com/drive/folders/19BqQzdCniXjI6STe7RGyj6MPzowULL3R?fbclid=IwAR2mfb2mZHE3Pqo6nqdzr9194rhrC593F82ZKuUQC3LaAcJT-hgvhTmacmo
Drive
Search an Drive
Shared with
0+
Files
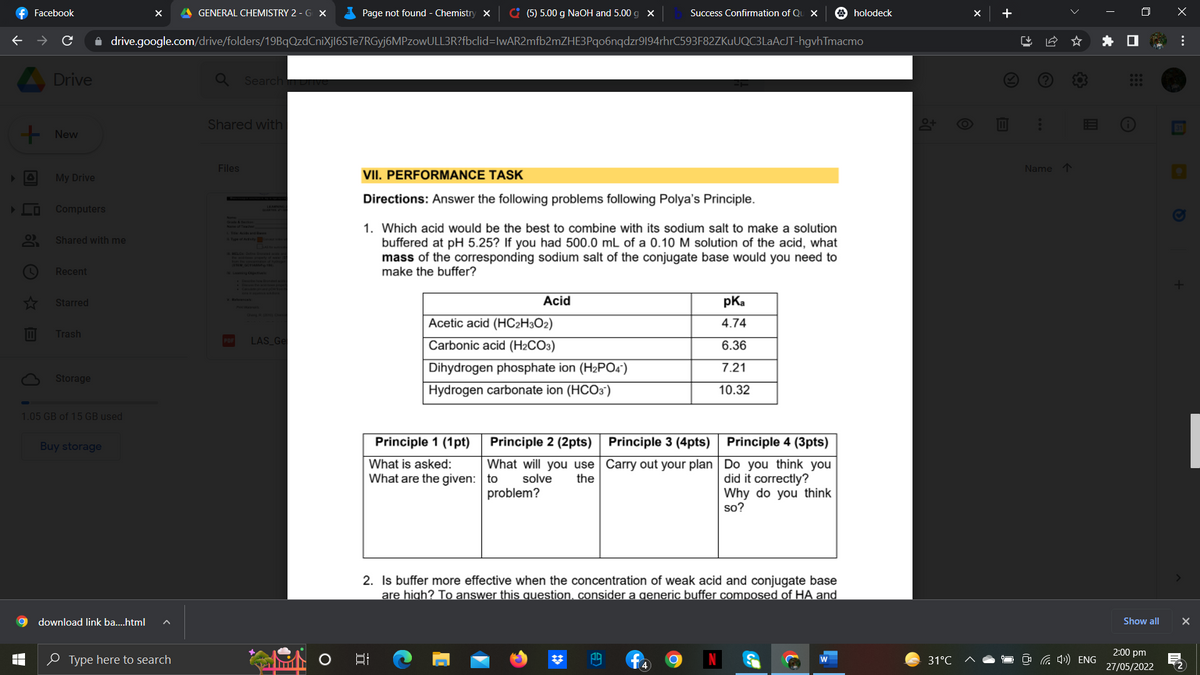
VII. PERFORMANCE TASK
Directions: Answer the following problems following Polya's Principle.
DUNNINGE
1. Which acid would be the best to combine with its sodium salt to make a solution
buffered at pH 5.25? If you had 500.0 mL of a 0.10 M solution of the acid, what
mass of the corresponding sodium salt of the conjugate base would you need to
make the buffer?
Acid
pKa
4.74
Acetic acid (HC2H3O2)
LAS_Ge
Carbonic acid (H2CO3)
6.36
7.21
Dihydrogen phosphate ion (H₂PO4)
Hydrogen carbonate ion (HCO3)
10.32
Principle 1 (1pt)
What is asked:
What are the given:
Principle 2 (2pts)
What will you use
to solve the
problem?
Principle 3 (4pts)
Carry out your plan
Principle 4 (3pts)
Do you think you
did it correctly?
Why do you think
so?
2. Is buffer more effective when the concentration of weak acid and conjugate base
are high? To answer this question, consider a generic buffer composed of HA and
발
PR
O
+ New
My Drive
Computers
Shared with me
Recent
Starred
Trash
Storage
1.05 GB of 15 GB used
Buy storage
download link ba....html A
Type here to search
(
▬
LI
31°C
+
↓
Name ↑
(4) ENG
I
Show all
2:00 pm
27/05/2022
x
...
+
|
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

