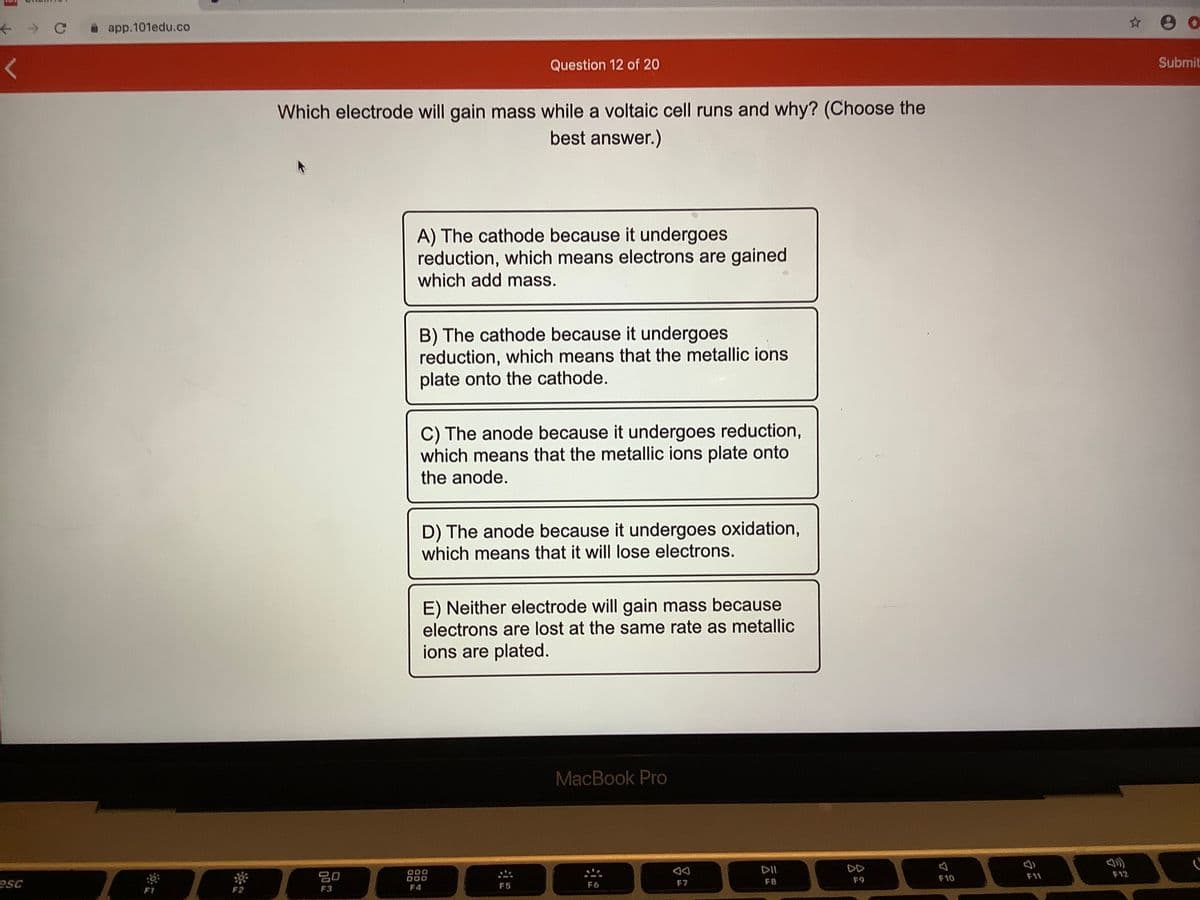
Which electrode will gain mass while a voltaic cell runs and why? (Choose the best answer.) A) The cathode because it undergoes reduction, which means electrons are gained which add mass. B) The cathode because it undergoes reduction, which means that the metallic ions plate onto the cathode. C) The anode because it undergoes reduction, which means that the metallic ions plate onto the anode. D) The anode because it undergoes oxidation, which means that it will lose electrons. E) Neither electrode will gain mass because electrons are lost at the same rate as metallic ions are plated.
Which electrode will gain mass while a voltaic cell runs and why? (Choose the best answer.) A) The cathode because it undergoes reduction, which means electrons are gained which add mass. B) The cathode because it undergoes reduction, which means that the metallic ions plate onto the cathode. C) The anode because it undergoes reduction, which means that the metallic ions plate onto the anode. D) The anode because it undergoes oxidation, which means that it will lose electrons. E) Neither electrode will gain mass because electrons are lost at the same rate as metallic ions are plated.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 42E
Related questions
Question
100%
Transcribed Image Text:←山 C
a app.101edu.co
Question 12 of 20
Submit
Which electrode will gain mass while a voltaic cell runs and why? (Choose the
best answer.)
A) The cathode because it undergoes
reduction, which means electrons are gained
which add mass.
B) The cathode because it undergoes
reduction, which means that the metallic ions
plate onto the cathode.
C) The anode because it undergoes reduction,
which means that the metallic ions plate onto
the anode.
D) The anode because it undergoes oxidation,
which means that it will lose electrons.
E) Neither electrode will gain mass because
electrons are lost at the same rate as metallic
ions are plated.
MacBook Pro
DD
吕口
888
000
F10
F11
F12
F9
esc
F5
F6
F7
F8
F1
F3
F4
002
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning