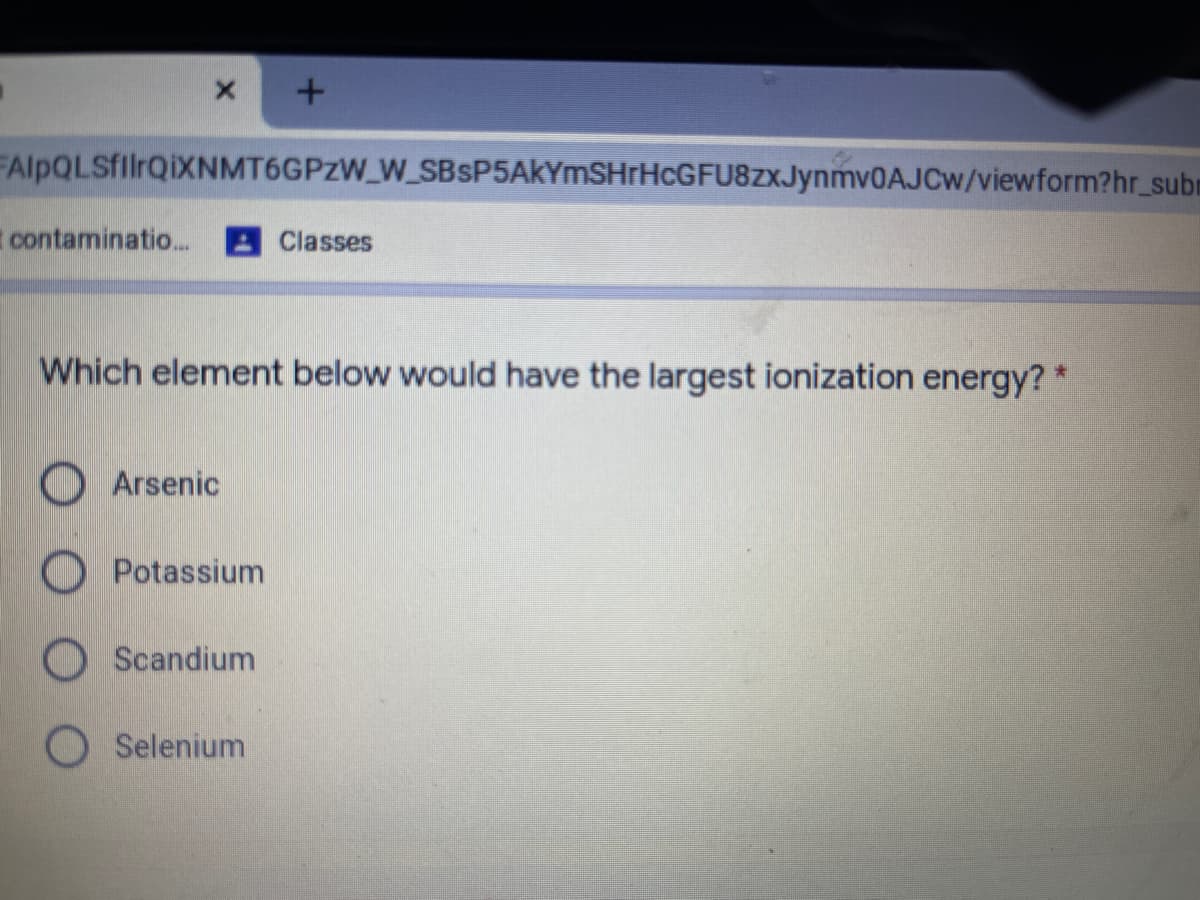
Ionization enthalpy:
It is the minimum amount of energy required to remove an electron of an atom or ion its gaseous phase.
It is measured in kJ/mole or eV.
Periodic table follows a trend for ionization energy in which the ionization energy increases moving from left to right in a period. In a period the electrons enter the same shell by increase in atomic number. Number of protons increases while the increases electrons are present in the same shell that results in decreasing atomic radius as the electrons are attracted much stronger by the nucleus.
The elements given belongs to period 4.
Selenium is present towards the right side of periodic table among the given elements.
So Selenium has the largest ionization energy.
Step by step
Solved in 2 steps









