Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 58AP
Related questions
Question
100%
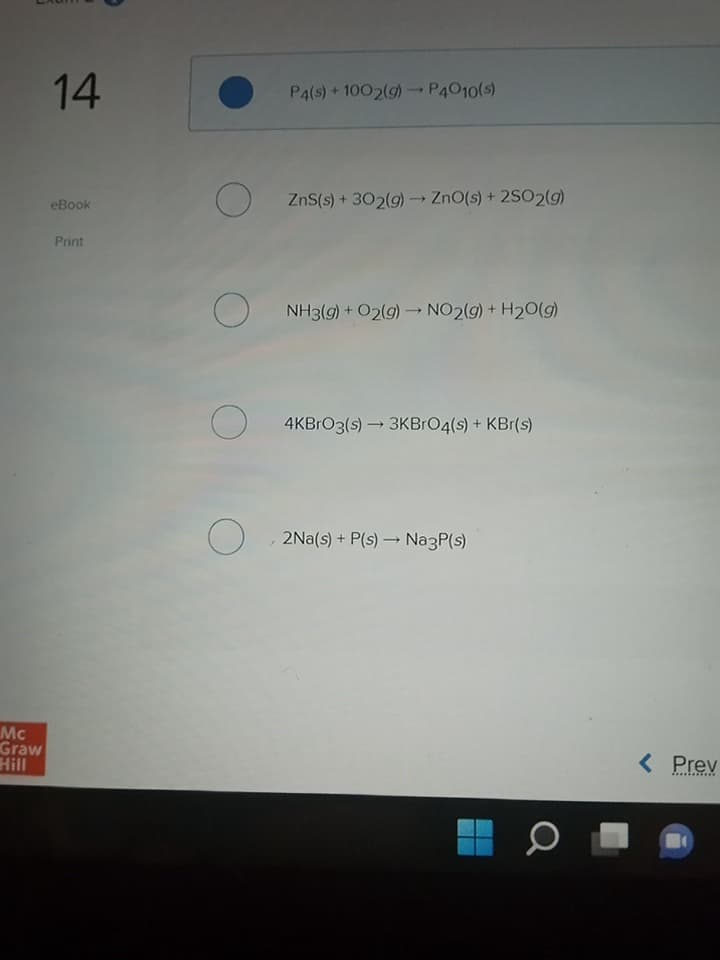
Which equation is balanced?
Transcribed Image Text:Mc
Graw
Hill
14
eBook
Print
O
1
P4(s) + 1002(g) →→ P4010(s)
-
ZnS(s) + 302(g) → ZnO(s) + 2SO2(g)
NH3(g) + O2(g) →→ NO2(g) + H₂O(g)
4KBrO3(s)→ 3KBrO4(s) + KBr(s)
O, 2Na(s) + P(s) → Na3P(s)
0
< Prev
HO
Transcribed Image Text:53°F
Cloudy
Mc
Graw
Hill
2
14
#
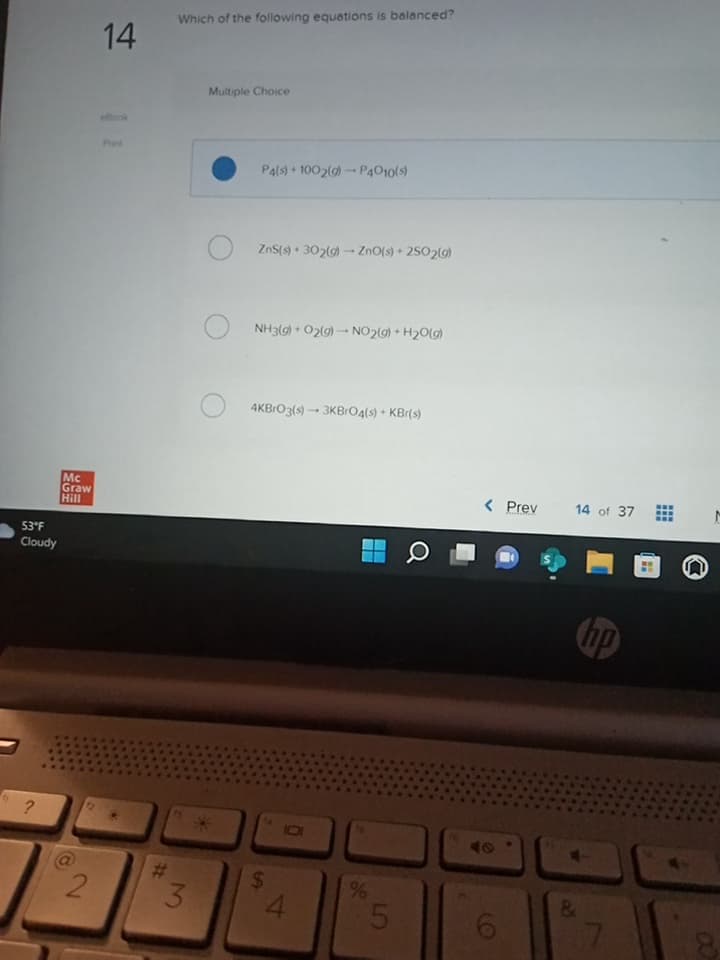
Which of the following equations is balanced?
3
Multiple Choice
P4(s) + 1002(g)-P4010(s)
ZnS(s) + 302(g) → ZnO(s) +2502(g)
NH3(g) + O₂(g) → NO2(g) + H₂O(g)
4KBrO3(s)-3KBrO4(s) + KBr(s)
$
4
%
5
< Prev
14 of 37
&
no
H
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning