Which of the following balanced chemical equations pertains to the chemical reaction involved in Procedure 13 СаО + H20 - Ca(ОН)2 Сао + H,о - 20a(ОН) 2 B c) 2 СаО + H20 Са(ОН)2 (D) 3 Сао + Нао - 3 Са(ОН)2
Which of the following balanced chemical equations pertains to the chemical reaction involved in Procedure 13 СаО + H20 - Ca(ОН)2 Сао + H,о - 20a(ОН) 2 B c) 2 СаО + H20 Са(ОН)2 (D) 3 Сао + Нао - 3 Са(ОН)2
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 62QAP: When corn is allowed to ferment, the fructose in the corn is converted to ethyl alcohol according to...
Related questions
Question
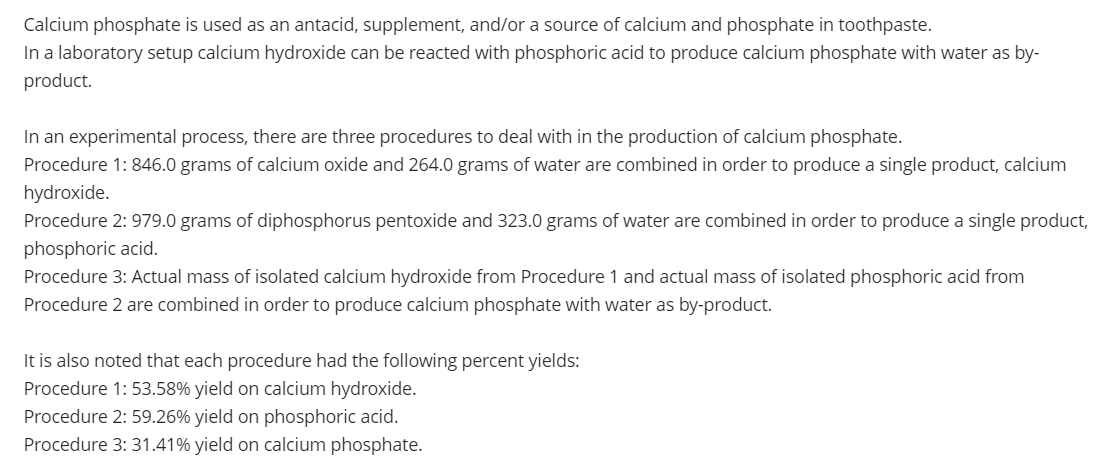
Transcribed Image Text:Calcium phosphate is used as an antacid, supplement, and/or a source of calcium and phosphate in toothpaste.
In a laboratory setup calcium hydroxide can be reacted with phosphoric acid to produce calcium phosphate with water as by-
product.
In an experimental process, there are three procedures to deal with in the production of calcium phosphate.
Procedure 1: 846.0 grams of calcium oxide and 264.0 grams of water are combined in order to produce a single product, calcium
hydroxide.
Procedure 2: 979.0 grams of diphosphorus pentoxide and 323.0 grams of water are combined in order to produce a single product,
phosphoric acid.
Procedure 3: Actual mass of isolated calcium hydroxide from Procedure 1 and actual mass of isolated phosphoric acid from
Procedure 2 are combined in order to produce calcium phosphate with water as by-product.
It is also noted that each procedure had the following percent yields:
Procedure 1: 53.58% yield on calcium hydroxide.
Procedure 2: 59.26% yield on phosphoric acid.
Procedure 3: 31.41% yield on calcium phosphate.
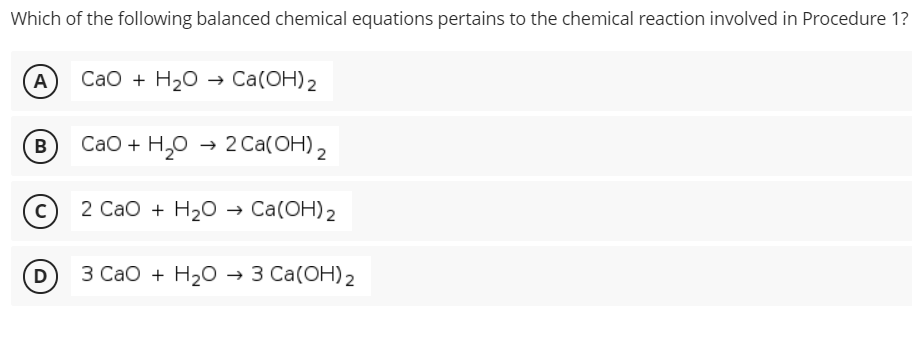
Transcribed Image Text:Which of the following balanced chemical equations pertains to the chemical reaction involved in Procedure 1?
A
СаО + H20 > Caа(ОН) 2
В
СаО + H,о - 20a(ОН) 2
2 СаО + H20 Са(ОН)2
D
3 Сао + H20 3 Cа(ОН)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning