Which of the following best explains why solubility generally increases as the temperature is increased? A The solute molecules gain energy and escapes from the surface of the solvent as temperature is increased. The entropy of the system increases as the temperature of the system is raised. At high temperature, bonds of solute molecules are broken down to form new bonds with the solvent molecules. The interaction of solute-solute and solvent-solvent becomes weak at high temperature resulting a higher chance for solute-solvent D interaction to occur.
Which of the following best explains why solubility generally increases as the temperature is increased? A The solute molecules gain energy and escapes from the surface of the solvent as temperature is increased. The entropy of the system increases as the temperature of the system is raised. At high temperature, bonds of solute molecules are broken down to form new bonds with the solvent molecules. The interaction of solute-solute and solvent-solvent becomes weak at high temperature resulting a higher chance for solute-solvent D interaction to occur.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.4QE
Related questions
Question
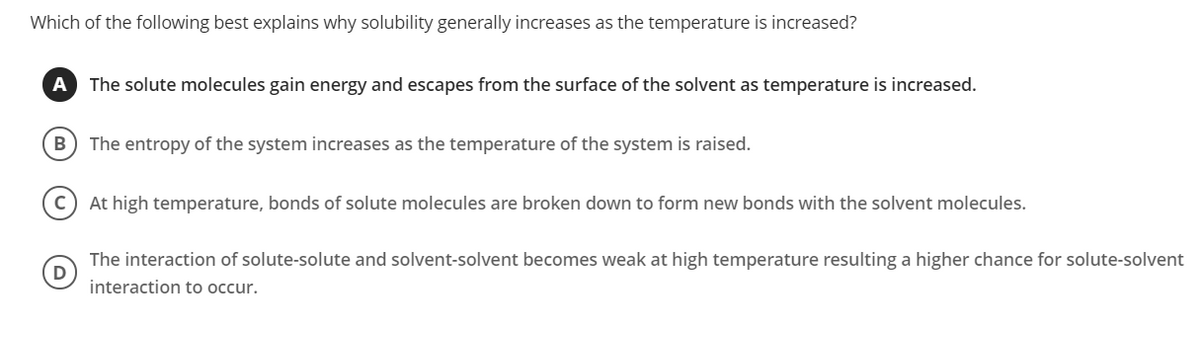
Transcribed Image Text:Which of the following best explains why solubility generally increases as the temperature is increased?
A
The solute molecules gain energy and escapes from the surface of the solvent as temperature is increased.
B
The entropy of the system increases as the temperature of the system is raised.
At high temperature, bonds of solute molecules are broken down to form new bonds with the solvent molecules.
The interaction of solute-solute and solvent-solvent becomes weak at high temperature resulting a higher chance for solute-solvent
interaction to occur.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning