Match the term with the related statement. Amixture with particles that settle out if undisturbed. The visible erratic motion of colloid particles, under a microscope. A measure of how much solute is dissolved in a specific amount of solvent or solution. The overall energy change that occurs when a solution forms. A heterogeneous mixture of intermediate size particles. The process of surrounding solute particles with solvent particles. A homogeneous mixture with particles less than 1 nm in diameter whose solute cannot be filtered out or settled out of solution. The scattering of light by dispersed colloid particles. : colloid : Tyndall effect : suspension :: solvation : heat of solution : concentration : solution : Brownian motion
Match the term with the related statement. Amixture with particles that settle out if undisturbed. The visible erratic motion of colloid particles, under a microscope. A measure of how much solute is dissolved in a specific amount of solvent or solution. The overall energy change that occurs when a solution forms. A heterogeneous mixture of intermediate size particles. The process of surrounding solute particles with solvent particles. A homogeneous mixture with particles less than 1 nm in diameter whose solute cannot be filtered out or settled out of solution. The scattering of light by dispersed colloid particles. : colloid : Tyndall effect : suspension :: solvation : heat of solution : concentration : solution : Brownian motion
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 6RQ: In terms of Raoults law, distinguish between an ideal liquid-liquid solution and a nonideal...
Related questions
Question
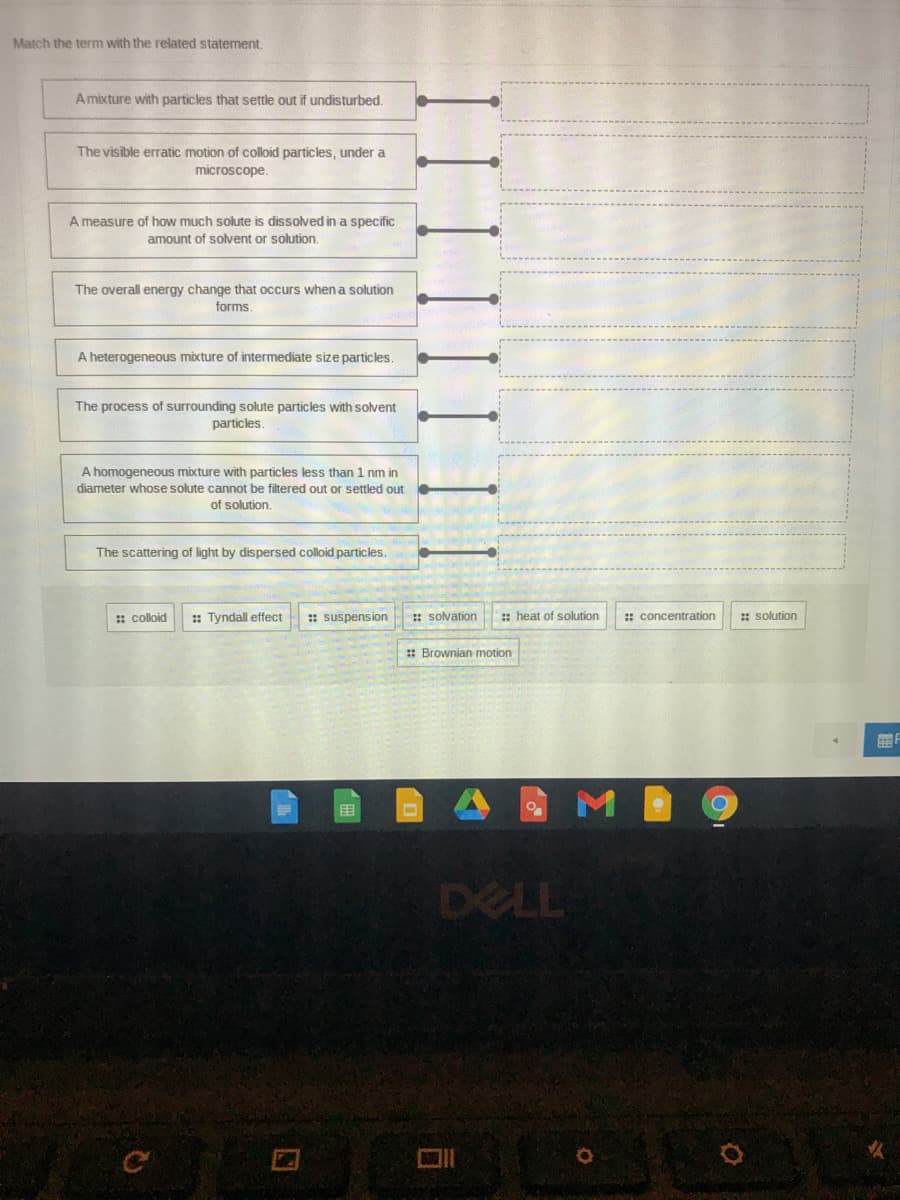
Transcribed Image Text:Match the term with the related statement,
A mixture with particles that settle out if undisturbed.
The visible erratic motion of colloid particles, under a
microscope.
A measure of how much solute is dissolved in a specific
amount of solvent or solution.
The overall energy change that occurs when a solution
forms.
A heterogeneous mixture of intermediate size particles.
The process of surrounding solute particles with solvent
particles.
A homogeneous mixture with particles less than 1 nm in
diameter whose solute cannot be filtered out or settled out
of solution.
The scattering of light by dispersed colloid particles.
: colloid
:: Tyndall effect
: suspension
: solvation
: heat of solution
: concentration
: solution
: Brownian motion
目
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning