Which of the following explains why alpha-amino acids, the building blocks of proteins, are nearly 1000 times more acidic than aliphatic carboxylic acids? H3N, R OH OH R An a-amino acid An aliphatic acid pk, = 5 pK, = 2 O Inductive effects of the aliphatic group destabilize the conjugate base. O Inductive effects of the amino group help stabilize the conjugate base. O Inductive effects of the aliphatic groups help stbilize the conjugate base. O Inductive effects of the amino group destabilize the conjugate base.
Which of the following explains why alpha-amino acids, the building blocks of proteins, are nearly 1000 times more acidic than aliphatic carboxylic acids? H3N, R OH OH R An a-amino acid An aliphatic acid pk, = 5 pK, = 2 O Inductive effects of the aliphatic group destabilize the conjugate base. O Inductive effects of the amino group help stabilize the conjugate base. O Inductive effects of the aliphatic groups help stbilize the conjugate base. O Inductive effects of the amino group destabilize the conjugate base.
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
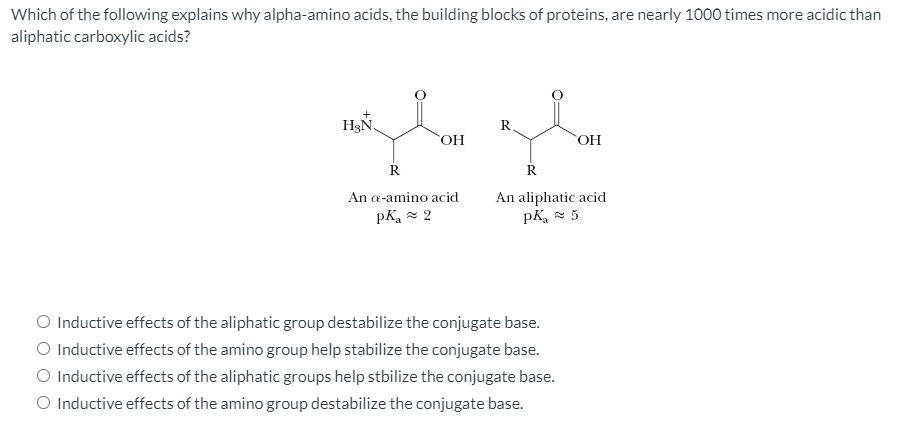
Transcribed Image Text:Which of the following explains why alpha-amino acids, the building blocks of proteins, are nearly 1000 times more acidic than
aliphatic carboxylic acids?
H3N.
R.
An a-amino acid
An aliphatic acid
pK, 5
pKa = 2
O Inductive effects of the aliphatic group destabilize the conjugate base.
O Inductive effects of the amino group help stabilize the conjugate base.
O Inductive effects of the aliphatic groups help stbilize the conjugate base.
O Inductive effects of the amino group destabilize the conjugate base.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you



Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning



Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning