Which of the following gases has the lowest rate of effusion? a a am hydogen holum H Не 10079 Inium 3 4.006 berylum boron carton nitrogon onygen fuonine neon 10 Li Be 6.941 sodium 90122 magneskm 12 10811 aluminium 13 12.011 scon 14.007 phosphona 15 15999 18.998 chorine 20.190 suur argon 16 17 18 Na Mg AI Si S CI Ar 24 305 caldum nickel 28 copper 29 26902 galum 31 28.006 gemankum 32 30.974 arsenic 32.065 selenium 34 35 453 bromine 39.948 krypon 36 scandum itanium vanadium chromium manganese 25 iron cobalt zine 20 21 22 23 24 26 27 30 33 35 K Ca Sc Ti Cr Mn Fe Co Ni Cu Zn Ga Ge As Se 40078 stontun 50.942 niobum 41 51.996 lybdenu lechnetum 42 65 39 cadmium 48 55.845 58.693 paladum 46 74.22 antinony 51 7896 leluum 44.956 54.908 58.903 hodum 63.546 silver 72.61 83 80 47 867 69723 79904 lodine yttrurn zconum ruthonum Indum in 38 39 40 43 44 45 47 49 50 52 53 54 Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Хе 91.224 hatnium 92 900 tantakum 95.94 tungsten 74 101.07 osmkum 106.42 platinum 78 10 gold 79 112.41 meroury 80 114.82 thalum 118.71 lead 121.76 bismuth 127 00 poknium 126 90 astatine 131.29 102.91 ndium barum lutem henium 56 57-70 71 72 73 75 76 77 81 82 83 85 86 Cs Ba Hf Ta W Re Os Ir TI Pb Bi Po At Rn 18384 seaborgium 106 190 23 hassium 200.59 ununbium 137.33 178 49 Lwrendum ruhorfordun dubnum 104 180 95 186 21 bohrum 192.22 208 98 174.97 196.08 metnerum ununnium 196.97 204.38 207.2 ununguadum 114 209 unununkum 88 89-102 103 105 107 108 109 110 111 112 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq 2611 pe lanthanum 57 cerum 58 praseodymun neodymium promethium 59 samarium 62 europum 63 gadoinum 64 lerbum 65 dysprosum oinum 66 67 thuum 69 ytterbum 70 ertium 60 61 68 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 138.91 actnim 89 144 24 157 25 aurtun 16250 167 26 140.12 140.91 149 neptunum 93 150.6 paonun 94 151.96 americum 158.93 berkelum 97 164.93 callomm estenium 99 168.93 fermum menevm nobelim 101 173.04 ** Actinide series 90 95 96 100 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 239.04 231 04 249 Select one: O a. SO2 O b. CO2 OC. F2 o d. N2 O e. CH4 е.
Which of the following gases has the lowest rate of effusion? a a am hydogen holum H Не 10079 Inium 3 4.006 berylum boron carton nitrogon onygen fuonine neon 10 Li Be 6.941 sodium 90122 magneskm 12 10811 aluminium 13 12.011 scon 14.007 phosphona 15 15999 18.998 chorine 20.190 suur argon 16 17 18 Na Mg AI Si S CI Ar 24 305 caldum nickel 28 copper 29 26902 galum 31 28.006 gemankum 32 30.974 arsenic 32.065 selenium 34 35 453 bromine 39.948 krypon 36 scandum itanium vanadium chromium manganese 25 iron cobalt zine 20 21 22 23 24 26 27 30 33 35 K Ca Sc Ti Cr Mn Fe Co Ni Cu Zn Ga Ge As Se 40078 stontun 50.942 niobum 41 51.996 lybdenu lechnetum 42 65 39 cadmium 48 55.845 58.693 paladum 46 74.22 antinony 51 7896 leluum 44.956 54.908 58.903 hodum 63.546 silver 72.61 83 80 47 867 69723 79904 lodine yttrurn zconum ruthonum Indum in 38 39 40 43 44 45 47 49 50 52 53 54 Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Хе 91.224 hatnium 92 900 tantakum 95.94 tungsten 74 101.07 osmkum 106.42 platinum 78 10 gold 79 112.41 meroury 80 114.82 thalum 118.71 lead 121.76 bismuth 127 00 poknium 126 90 astatine 131.29 102.91 ndium barum lutem henium 56 57-70 71 72 73 75 76 77 81 82 83 85 86 Cs Ba Hf Ta W Re Os Ir TI Pb Bi Po At Rn 18384 seaborgium 106 190 23 hassium 200.59 ununbium 137.33 178 49 Lwrendum ruhorfordun dubnum 104 180 95 186 21 bohrum 192.22 208 98 174.97 196.08 metnerum ununnium 196.97 204.38 207.2 ununguadum 114 209 unununkum 88 89-102 103 105 107 108 109 110 111 112 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq 2611 pe lanthanum 57 cerum 58 praseodymun neodymium promethium 59 samarium 62 europum 63 gadoinum 64 lerbum 65 dysprosum oinum 66 67 thuum 69 ytterbum 70 ertium 60 61 68 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 138.91 actnim 89 144 24 157 25 aurtun 16250 167 26 140.12 140.91 149 neptunum 93 150.6 paonun 94 151.96 americum 158.93 berkelum 97 164.93 callomm estenium 99 168.93 fermum menevm nobelim 101 173.04 ** Actinide series 90 95 96 100 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 239.04 231 04 249 Select one: O a. SO2 O b. CO2 OC. F2 o d. N2 O e. CH4 е.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 94QAP
Related questions
Question
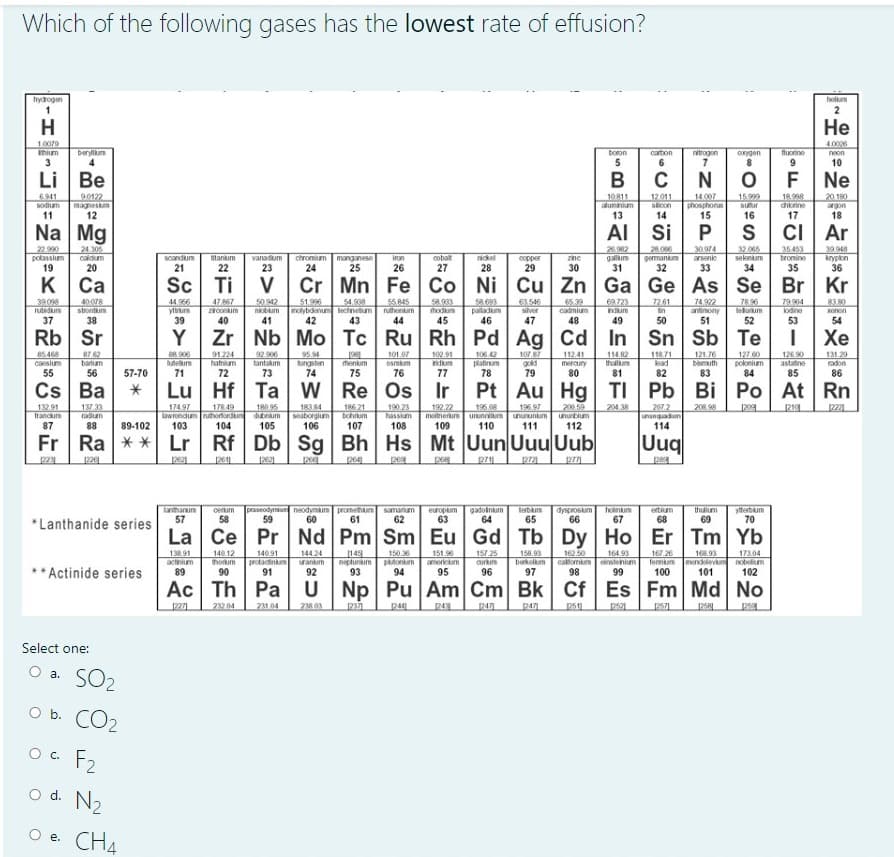
Transcribed Image Text:Which of the following gases has the lowest rate of effusion?
hydogen
helum
H
Не
4006
10079
Inium
3
berylum
boron
carton
nitrogon
onygen
fuonine
neon
10
Li Be
6.941
sodium
11
90122
magneskm
12
10811
luminium
13
14.007
phosphona
15
15999
18.998
20.180
12.011
scon
suur
chorine
argon
16
17
18
Na Mg
AI Si
P
S
CI Ar
22990
potassium
19
24 305
caldum
vanadium
23
nickel
28
copper
29
26902
galum
31
28.006
gemankum
32
30974
arsenie
32.065
selenium
34
35453
bromine
39.948
krypon
36
scandum
itanium
chromium
manganese
iron
cobalt
zine
20
21
22
24
25
26
27
30
33
35
K Ca
Sc Ti
Cr Mn Fe
Co Ni Cu Zn Ga Ge As Se
Br Kr
39.098
rutidum
40078
stontun
38
51.996
lybdenum lechnetum
42
65 39
cadmium
48
55.845
58.693
paladum
46
44.956
47.867
54.908
58.903
hodun
45
69723
72.61
83 80
50.942
niobum
41
63.546
silver
7896
teluum
79904
lodine
yttrurn
zconum
ruthenum
Indum
in
antinony
51
39
40
43
44
47
49
50
52
53
54
Rb Sr
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In Sn Sb Te
Хе
91.224
hafnium
72
92 900
tantakum
95.54
tungsten
74
101.07
osmkum
106.42
platinum
78
10
gold
79
112.41
meroury
80
114.82
thalium
81
118.71
lead
121.76
bismuth
127 00
poknium
126 90
astatine
131.29
102.91
ndium
barum
luteim
henium
75
56
57-70
71
73
76
77
82
83
85
86
Cs Ba
Hf Ta W Re Os Ir
TI Pb Bi Po At Rn
183 4
seaborgium
106
190 23
hassium
208 98
178 49
Lwrendum ruhorfordun dubnum
104
180 95
186 21
bohrum
192.22
19608
motnerum ununnium
200.59
ununbium
207.2
ununguadum
114
137.33
174.97
196.97
204.38
209
21g
unuunkum
88
89-102
103
105
107
108
109
110
111
112
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
pr
anhanum
57
cerum
58
praseodyrmun neodymum peomethum
60
samarium
62
europum
63
gadolnum
64
lerbaum
65
dysprosum oinum
66
67
ertium
68
thuum
69
ytterbum
70
59
61
*Lanthanide series
La
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
138.91
actnim
89
144 24
157 25
aurkun
162.50
167 26
140.12
140.91
149
neptuntum
93
150.36
ponum
94
151.96
americum
158.93
berkelum
164.93
callomkm estenium
99
168.93
fermum mendevm nobelim
101
173.04
uranum
** Actinide series
90
95
96
97
100
102
Ac Th Pa U Np Pu Am Cm Bk Cf
Es Fm Md No
23.04
231 04
249
P47
P511
Select one:
O a.
SO2
O b. CO2
OC.
F2
o d. N2
O e. CH4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
