Which of the following is a complete balanced reaction for an Arrhenius base in water? NaOH (s) 2 NaH (s) + O2 (g) →> ○ Mg(OH) 2 (s) → Mg2+ (aq) + OH (aq) ○ HNO2 (aq) → H+ (aq) + NO₂ (aq) KOH (s) →> K+ (aq) + OH¯ (aq)
Which of the following is a complete balanced reaction for an Arrhenius base in water? NaOH (s) 2 NaH (s) + O2 (g) →> ○ Mg(OH) 2 (s) → Mg2+ (aq) + OH (aq) ○ HNO2 (aq) → H+ (aq) + NO₂ (aq) KOH (s) →> K+ (aq) + OH¯ (aq)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 66QRT: Complete each of these reactions by filling in the blanks. Predict whether each reaction is...
Related questions
Question
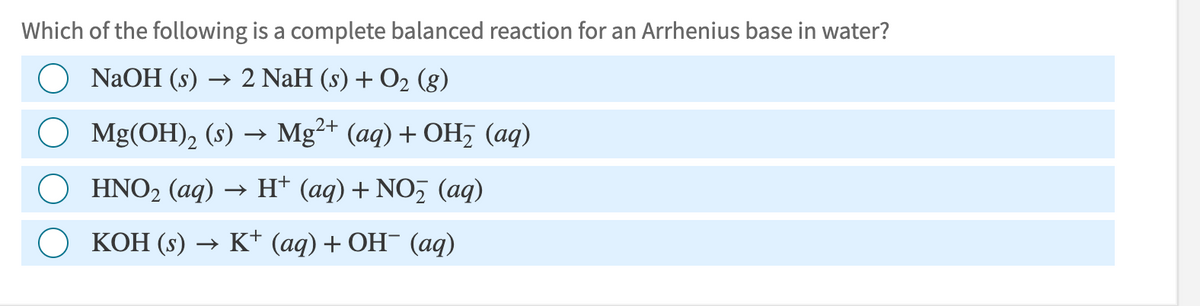
Transcribed Image Text:Which of the following is a complete balanced reaction for an Arrhenius base in water?
NaOH (s) 2 NaH (s) + O2 (g)
→>
○ Mg(OH) 2 (s) → Mg2+ (aq) + OH (aq)
○ HNO2 (aq) → H+ (aq) + NO₂ (aq)
KOH (s) →> K+ (aq) + OH¯ (aq)
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning